J Korean Med Sci.
2024 Apr;39(12):e119. 10.3346/jkms.2024.39.e119.
Twenty Years of Progress and the Way Forward: Immunization Registry Information System in Korea
- Affiliations
-
- 1Division of Immunization, Korea Disease Control and Prevention Agency, Cheongju, Korea
- 2Graduate School of Public Health, Chungnam National University, Daejeon, Korea
- 3KDI School of Public Policy and Management, Sejong, Korea
- KMID: 2554212
- DOI: http://doi.org/10.3346/jkms.2024.39.e119
Abstract
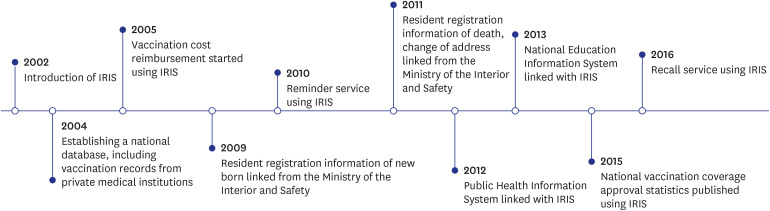
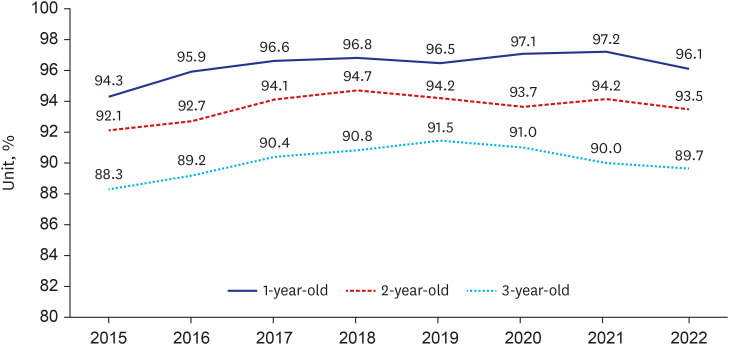
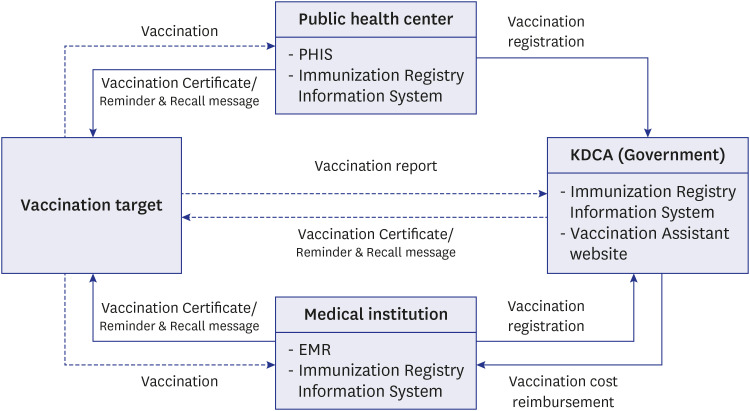
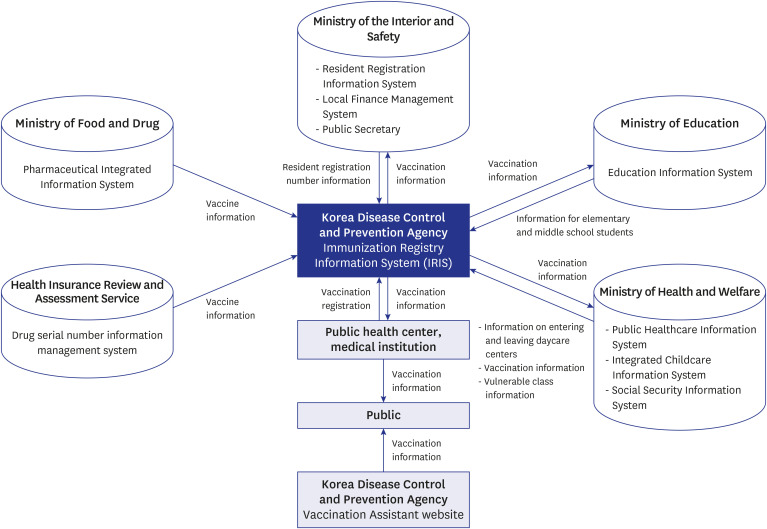
- The National Immunization Program in The Republic of Korea offers mandatory and free vaccinations to children under 12, regulated by the Infectious Disease Prevention and Control Act. Tracking vaccination coverage is crucial for population protection and public health strategies. Since 2002, the Immunization Registry Information System (IRIS) has been used nationwide to capture vaccination data. This study reviewed documents related to IRIS’s establishment and development. The Republic of Korea legally supports IRIS's construction and data collection, integrating vaccination data with the Ministry of the Interior and Safety's resident registration to minimize errors. This collaboration also facilitates cost reimbursement and digital registration, promoting wider vaccination coverage. IRIS manages expense claims once vaccination details are logged, and authorized medical institutions can access these records in real-time. Since 2015, the Korea Disease Control and Prevention Agency has been compiling annual data on national vaccination coverage. IRIS also sends automated reminders in 12 languages, reports adverse effects, and issues vaccination certificates. However, IRIS lacks integration between vaccine and disease registries, unlike countries such as England, Denmark, and the Netherlands. Improving integration capabilities could enhance IRIS's support for public health through an integrated information system.
Keyword
Figure
Reference
-
1. Getchell M, Mantaring EJ, Yee K, Pronyk P. Cost-effectiveness of sub-national geographically targeted vaccination programs: a systematic review. Vaccine. 2023; 41(14):2320–2328. PMID: 36781333.2. Sun ZW, Fu Y, Lu HL, Yang RX, Goyal H, Jiang Y, et al. Association of rotavirus vaccines with reduction in rotavirus gastroenteritis in children younger than 5 years: a systematic review and meta-analysis of randomized clinical trials and observational studies. JAMA Pediatr. 2021; 175(7):e210347. PMID: 33970192.3. Ozawa S, Mirelman A, Stack ML, Walker DG, Levine OS. Cost-effectiveness and economic benefits of vaccines in low- and middle-income countries: a systematic review. Vaccine. 2012; 31(1):96–108. PMID: 23142307.4. Leidner AJ, Murthy N, Chesson HW, Biggerstaff M, Stoecker C, Harris AM, et al. Cost-effectiveness of adult vaccinations: a systematic review. Vaccine. 2019; 37(2):226–234. PMID: 30527660.5. Ting EE, Sander B, Ungar WJ. Systematic review of the cost-effectiveness of influenza immunization programs. Vaccine. 2017; 35(15):1828–1843. PMID: 28284681.6. Olivera Mesa D, Winskill P, Ghani AC, Hauck K. The societal cost of vaccine refusal: a modelling study using measles vaccination as a case study. Vaccine. 2023; 41(28):4129–4137. PMID: 37263873.7. Ghaznavi C, Eguchi A, Suu Lwin K, Yoneoka D, Tanoue Y, Kumar Rauniyar S, et al. Estimating global changes in routine childhood vaccination coverage during the COVID-19 pandemic, 2020–2021. Vaccine. 2023; 41(28):4151–4157. PMID: 37246068.8. Moon D, Kim S, Kim MH, Jeong D, Choi H. Contracting out national immunization program does not improve vaccination rate nor socioeconomic inequality: a case study of seasonal influenza vaccination in South Korea. Front Public Health. 2021; 9:769176. PMID: 34805079.9. Park B, Choi EJ, Park B, Han H, Cho SJ, Choi HJ, et al. Factors influencing vaccination in Korea: findings from focus group interviews. J Prev Med Public Health. 2018; 51(4):173–180. PMID: 30071704.10. Kim SJ, Kwon SL, Lee JY, Oh J, Kwon GY. Why school is crucial to increase vaccination coverage for children: evaluation of school vaccination check program in South Korea 2021–2022. Vaccine. 2023; 41(21):3380–3386. PMID: 37105889.11. Kim H. Establishment of evaluation system and priority setting of new vaccine introduction into national immunization program. Seoul: National Evidence-Based Healthcare Collaborating Agency;2020.12. Pebody R. Vaccine registers--experiences from Europe and elsewhere. Euro Surveill. 2012; 17(17):20159. PMID: 22551493.13. Baum U, Sundman J, Jääskeläinen S, Nohynek H, Puumalainen T, Jokinen J. Establishing and maintaining the national vaccination register in Finland. Euro Surveill. 2017; 22(17):30520. PMID: 28488994.14. Grove Krause T, Jakobsen S, Haarh M, Mølbak K. The Danish vaccination register. Euro Surveill. 2012; 17(17):20155. PMID: 22551494.15. Trogstad L, Ung G, Hagerup-Jenssen M, Cappelen I, Haugen IL, Feiring B. The Norwegian immunisation register--SYSVAK. Euro Surveill. 2012; 17(16):20147. PMID: 22551462.16. Chrapkowska C, Galanis I, Kark M, Lepp T, Lindstrand A, Roth A, et al. Validation of the new Swedish vaccination register - accuracy and completeness of register data. Vaccine. 2020; 38(25):4104–4110. PMID: 32359874.17. Lee MS, Kim EY, Lee KS, Lee SG, Hong JY, Kim KY, et al. Environment and infrastructure for development of national immunization electronic registry in private clinical practice setting in Korea. J Korean Soc Matern Child Health. 2003; 7(2):193–206.18. Hanney SR, Gonzalez-Block MA, Buxton MJ, Kogan M. The utilisation of health research in policy-making: concepts, examples and methods of assessment. Health Res Policy Syst. 2003; 1(1):2. PMID: 12646071.19. Amirthalingam G, White J, Ramsay M. Measuring childhood vaccine coverage in England: the role of child health information systems. Euro Surveill. 2012; 17(16):20149. PMID: 22551461.20. Bernal-González PJ, Navarro-Alonso JA, Pérez-Martin JJ. Computerised vaccination register for the Murcia region, Spain, 1991 to 2011. Euro Surveill. 2012; 17(16):20150. PMID: 22551463.21. Alfonsi V, D’Ancona F, Rota MC, Giambi C, Ranghiasci A, Iannazzo S, et al. Immunisation registers in Italy: a patchwork of computerisation. Euro Surveill. 2012; 17(17):20156. PMID: 22551498.22. Lee SG, Jeon SY, Oh HK. Qualitative views in computerized registration coverage by different cost supports for national immunization program. J Korean Soc Matern Child Health. 2012; 16(1):113–121.23. Kim CS, Park O, Kim MY, Kim MJ, Lee SG, Jung HK. A study on registration data analysis of national immunization registry information system. J Korea Inst Inf Commun Eng. 2015; 19(5):1151–1156.24. June Choe Y, Yi S, Hwang I, Kim J, Park YJ, Cho E, et al. Safety and effectiveness of BNT162b2 mRNA Covid-19 vaccine in adolescents. Vaccine. 2022; 40(5):691–694. PMID: 35012777.25. Eom J, Kim J. Vaccine policy and judicial justice: from immunity passport to vaccine pass. Hum Beings Environ Their Future. 2022; (28):31–63.26. Kwon SL, Oh J. COVID-19 vaccination program in South Korea: a long journey toward a new normal. Health Policy Technol. 2022; 11(2):100601. PMID: 35127400.27. Lee SG, Jeon SY, Go UY, Kim MJ, Lee SH. Evaluation of data error, completeness, timeliness of national immunization registry in Korea. J Korean Soc Matern Child Health. 2009; 13(2):135–144.28. Nam HJ, Lee SG, Jeon SY, Om JE, Park KS. Investigation of children with no vaccinations recorded on the national immunization registry information system. J Korean Soc Matern Child Health. 2017; 21(3):176–181.29. Lee J, Jeong H, Kim S, Yu J, Kim G. National childhood vaccination coverage among children aged 1–3 and 6 years in Korea, 2018. Public Health Wkly Rep. 2019; 12(39):1548–1558.30. Park JJ, Kim MJ, Kong IS. Quality control of registered immunization data. Public Health Wkly Rep. 2016; 9(43):866–869.31. Choe YJ, Yang JJ, Park SK, Choi EH, Lee HJ. Comparative estimation of coverage between national immunization program vaccines and non-NIP vaccines in Korea. J Korean Med Sci. 2013; 28(9):1283–1288. PMID: 24015031.32. Stephens AB, Wynn CS, Stockwell MS. Understanding the use of digital technology to promote human papillomavirus vaccination - a RE-AIM framework approach. Hum Vaccin Immunother. 2019; 15(7-8):1549–1561. PMID: 31158064.33. Jacobson Vann JC, Jacobson RM, Coyne-Beasley T, Asafu-Adjei JK, Szilagyi PG. Patient reminder and recall interventions to improve immunization rates. Cochrane Database Syst Rev. 2018; 1(1):CD003941. PMID: 29342498.34. Yu JH, Jeong HJ, Kim SJ, Lee JY, Choe YJ, Choi EH, et al. Sustained vaccination coverage during the coronavirus disease 2019 epidemic in the Republic of Korea. Vaccines (Basel). 2020; 9(1):2. PMID: 33375172.35. Siedler A, Rieck T, Reuss A, Walter D, Poggensee G, Poethko-Müller C, et al. Estimating vaccination coverage in the absence of immunisation registers--the German experience. Euro Surveill. 2012; 17(17):20152. PMID: 22551497.36. van Lier A, Oomen P, de Hoogh P, Drijfhout I, Elsinghorst B, Kemmeren J, et al. Præventis, the immunisation register of the Netherlands: a tool to evaluate the national immunisation programme. Euro Surveill. 2012; 17(17):20153. PMID: 22551495.37. Chin LK, Crawford NW, Rowles G, Buttery JP. Australian immunisation registers: established foundations and opportunities for improvement. Euro Surveill. 2012; 17(16):20148. PMID: 22551464.38. Park B, Lee YK, Cho LY, Go UY, Yang JJ, Ma SH, et al. Estimation of nationwide vaccination coverage and comparison of interview and telephone survey methodology for estimating vaccination status. J Korean Med Sci. 2011; 26(6):711–719. PMID: 21655054.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rotavirus Vaccine Coverage and Related Factors
- The Relationship between Retention of the Maternal Child Health Handbook, Awareness of DPT Additional Immunization and DPT Additional Immunization
- Computerzation of Radiation Oncology Practice Using Order-Communicating System
- Immunization Policy in Korea
- Investigation of Children with No Vaccinations Recorded on the National Immunization Registry Information System