Brain Tumor Res Treat.
2024 Jul;12(3):192-199. 10.14791/btrt.2024.0023.
Malignant Transformation of Meningioma With TERT Promoter Mutation: A Case Report
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Pathology, National Cancer Center, Goyang, Korea
- 3Department of Cancer Control, National Cancer Center, Graduate School of Cancer Science and Policy, Goyang, Korea
- KMID: 2558438
- DOI: http://doi.org/10.14791/btrt.2024.0023
Abstract
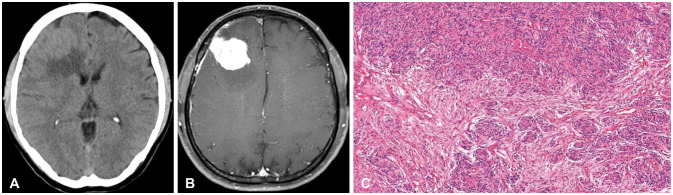
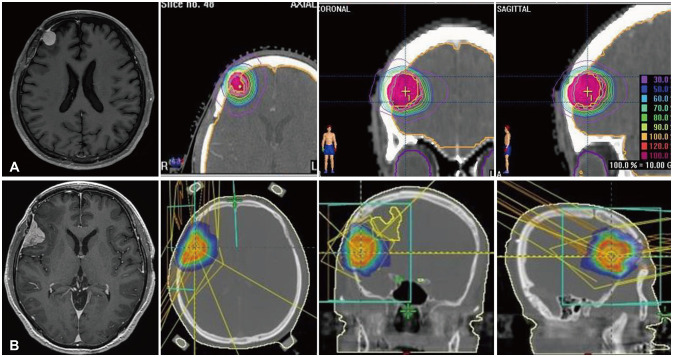
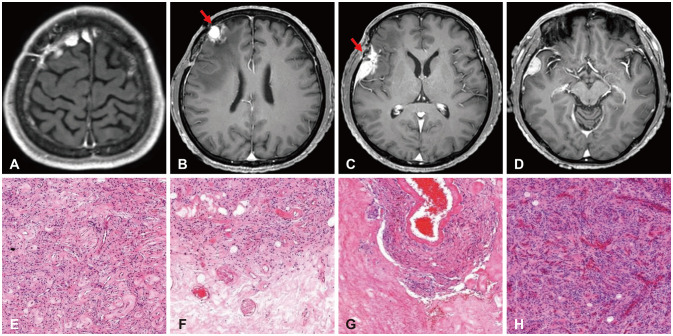
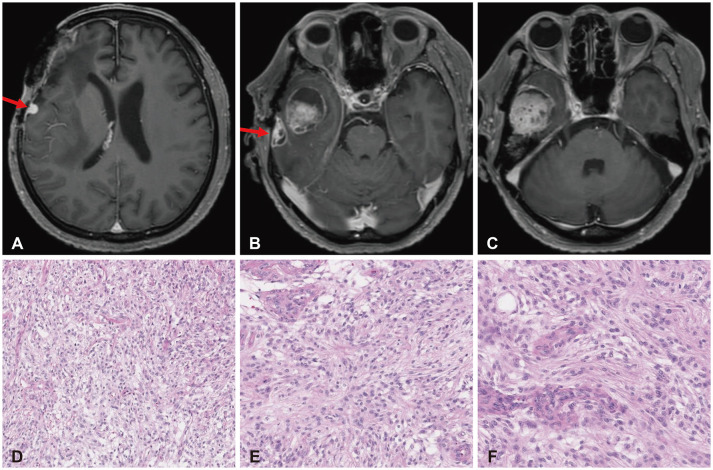
- High-grade meningiomas make up a relatively minor proportion of meningiomas, which are one of the most common types of primary intracranial tumors in adults. Though rare, a considerable portion of highgrade meningiomas arise from malignant transformation of benign meningiomas. The 2021 World Health Organization (WHO) classification criteria introduced molecular markers in the diagnosis and grading of central nervous system (CNS) tumors and assigned certain genomic mutations to grade 3 meningiomas. We report a case of a 54-year-old male patient who underwent stepwise malignant transformation of meningioma from WHO grade 1 to grade 3 within 10 years, during the course of five surgeries followed by adjuvant stereotactic radiosurgery and radiotherapy. We performed next-generation sequencing (NGS) on the most recent grade 3 meningioma specimen and found that it carried a telomerase reverse transcriptase promoter (TERTp) mutation (c.-124C>T) in accordance with the 2021 WHO criteria for grade 3 meningiomas. We then retrospectively examined the previous grade 1 and 2 specimens and found them to have the same mutation. We reviewed the significance of molecular markers in the diagnosis of meningiomas, possible genetic alterations associated with their malignant transformation, and what measures could be taken to effectively manage meningiomas considering NGS findings.
Keyword
Figure
Reference
-
1. Driver J, Hoffman SE, Tavakol S, Woodward E, Maury EA, Bhave V, et al. A molecularly integrated grade for meningioma. Neuro Oncol. 2022; 24:796–808. PMID: 34508644.2. Nakasu S, Nakasu Y, Fukami T, Jito J, Nozaki K. Growth curve analysis of asymptomatic and symptomatic meningiomas. J Neurooncol. 2011; 102:303–310. PMID: 20686821.3. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010; 99:307–314. PMID: 20821343.4. Wang J, Wang L, Luo B, Chen Z, Xiong Z, Fang M, et al. Recurrent meningioma with malignant transformation: a case report and literature review. Int J Clin Exp Med. 2015; 8:16845–16849. PMID: 26629232.5. Qu X, Jiang J, Wang H, Zhang C, Deng Q, Xu X, et al. Malignant transformation of meningioma: case report. Medicine (Baltimore). 2023; 102:e33409. PMID: 37000075.6. Jellinger K, Slowik F. Histological subtypes and prognostic problems in meningiomas. J Neurol. 1975; 208:279–298. PMID: 50413.7. Jääskeläinen J, Haltia M, Servo A. Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surg Neurol. 1986; 25:233–242. PMID: 3945904.8. Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016; 17:e383–e391. PMID: 27599143.9. Marciscano AE, Stemmer-Rachamimov AO, Niemierko A, Larvie M, Curry WT, Barker FG 2nd, et al. Benign meningiomas (WHO grade I) with atypical histological features: correlation of histopathological features with clinical outcomes. J Neurosurg. 2016; 124:106–114. PMID: 26274991.10. Sahm F, Schrimpf D, Olar A, Koelsche C, Reuss D, Bissel J, et al. TERT promoter mutations and risk of recurrence in meningioma. J Natl Cancer Inst. 2015; 108:djv377. PMID: 26668184.11. Sievers P, Hielscher T, Schrimpf D, Stichel D, Reuss DE, Berghoff AS, et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020; 140:409–413. PMID: 32642869.12. Gauchotte G, Peyre M, Pouget C, Cazals-Hatem D, Polivka M, Rech F, et al. Prognostic value of histopathological features and loss of H3K27me3 immunolabeling in anaplastic meningioma: a multicenter retrospective study. J Neuropathol Exp Neurol. 2020; 79:754–762. PMID: 32447376.13. Nassiri F, Mamatjan Y, Suppiah S, Badhiwala JH, Mansouri S, Karimi S, et al. DNA methylation profiling to predict recurrence risk in meningioma: development and validation of a nomogram to optimize clinical management. Neuro Oncol. 2019; 21:901–910. PMID: 31158293.14. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021; 23:1231–1251. PMID: 34185076.15. Nasrallah MP, Aldape KD. Molecular classification and grading of meningioma. J Neurooncol. 2023; 161:373–381. PMID: 36802047.16. Fahlström A, Dwivedi S, Drummond K. Multiple meningiomas: epidemiology, management, and outcomes. Neurooncol Adv. 2023; 5(Suppl 1):i35–i48. PMID: 37287575.17. Araújo Pereira BJ, Nogueira de Almeida A, Pires de Aguiar PH, Paiva WS, Teixeira MJ, Nagahashi Marie SK. Multiple intracranial meningiomas: a case series and review of the literature. World Neurosurg. 2019; 122:e1536–e1541. PMID: 30471445.18. Champeaux C, Wilson E, Shieff C, Khan AA, Thorne L. WHO grade II meningioma: a retrospective study for outcome and prognostic factor assessment. J Neurooncol. 2016; 129:337–345. PMID: 27311726.19. Ketter R, Henn W, Niedermayer I, Steilen-Gimbel H, König J, Zang KD, et al. Predictive value of progression-associated chromosomal aberrations for the prognosis of meningiomas: a retrospective study of 198 cases. J Neurosurg. 2001; 95:601–607. PMID: 11596954.20. Al-Mefty O, Kadri PA, Pravdenkova S, Sawyer JR, Stangeby C, Husain M. Malignant progression in meningioma: documentation of a series and analysis of cytogenetic findings. J Neurosurg. 2004; 101:210–218. PMID: 15309910.21. Harmancı AS, Youngblood MW, Clark VE, Coşkun S, Henegariu O, Duran D, et al. Integrated genomic analyses of de novo pathways underlying atypical meningiomas. Nat Commun. 2018; 9:16215. PMID: 29676392.22. Goutagny S, Nault JC, Mallet M, Henin D, Rossi JZ, Kalamarides M. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014; 24:184–189. PMID: 24261697.23. Juratli TA, Thiede C, Koerner MVA, Tummala SS, Daubner D, Shankar GM, et al. Intratumoral heterogeneity and TERT promoter mutations in progressive/higher-grade meningiomas. Oncotarget. 2017; 8:109228–109237. PMID: 29312603.24. Kwon SM, Kim JH, Yoo HJ, Kim YH, Hong SH, Cho YH, et al. Predictive factors for high-grade transformation in benign meningiomas. Clin Neurol Neurosurg. 2020; 195:105897. PMID: 32505062.25. Nakasu S, Notsu A, Na K, Nakasu Y. Malignant transformation of WHO grade I meningiomas after surgery or radiosurgery: systematic review and meta-analysis of observational studies. Neurooncol Adv. 2020; 2:vdaa129. PMID: 33305267.26. Maier AD, Meddis A, Mirian C, Haslund-Vinding J, Bartek J, Krog SM, et al. Gene expression analysis during progression of malignant meningioma compared to benign meningioma. J Neurosurg. 2022; 138:1302–1312. PMID: 36115056.27. Spiegl-Kreinecker S, Lötsch D, Neumayer K, Kastler L, Gojo J, Pirker C, et al. TERT promoter mutations are associated with poor prognosis and cell immortalization in meningioma. Neuro Oncol. 2018; 20:1584–1593. PMID: 30010853.28. Mirian C, Duun-Henriksen AK, Juratli T, Sahm F, Spiegl-Kreinecker S, Peyre M, et al. Poor prognosis associated with TERT gene alterations in meningioma is independent of the WHO classification: an individual patient data meta-analysis. J Neurol Neurosurg Psychiatry. 2020; 91:378–387. PMID: 32041819.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Early Recurrence of a Lateral Ventricle Meningioma with Malignant Transformation
- Meningioma with Multiple Recurrences and Malignant Transformation Differences in expression of MIB1, p53 and progesterone receptor
- TERT Promoter Mutations and Tumor Persistence/Recurrence in Papillary Thyroid Cancer
- Thyroid Cancer, Iodine, and Gene Mutation
- Mechanisms of TERT Reactivation and Its Interaction with BRAFV600E