Brain Tumor Res Treat.
2024 Jul;12(3):181-185. 10.14791/btrt.2024.0015.
Cerebrospinal Fluid Seeding Versus Inflammation in Setting of Ventriculoperitoneal Shunt as a Potential Cause for Distant Recurrence of Glioblastoma
- Affiliations
-
- 1Department of Neurological Surgery, University of Pittsburgh Medical Center, Pittsburgh, PA, USA
- 2Division of Anatomic Pathology, Department of Pathology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA
- KMID: 2558436
- DOI: http://doi.org/10.14791/btrt.2024.0015
Abstract
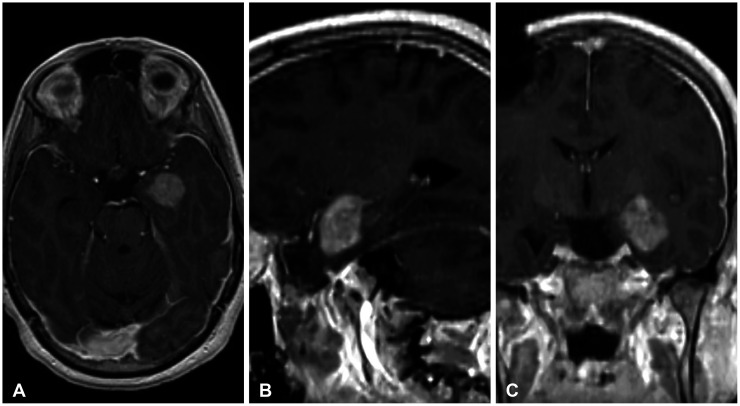
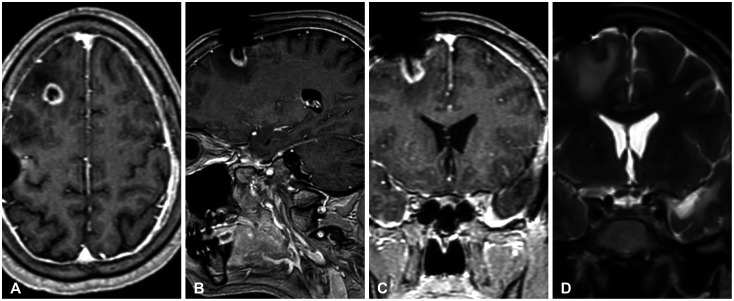
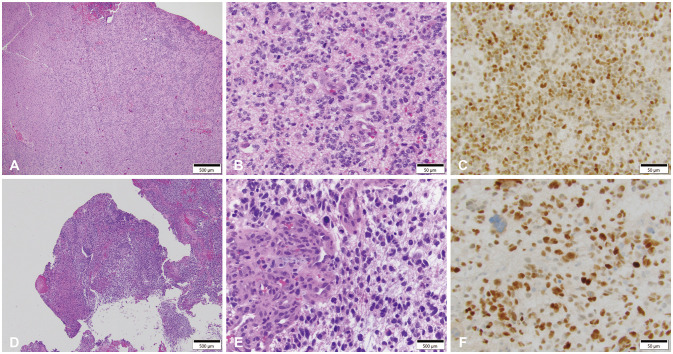
- Glioblastoma multiforme (GBM) is the most common primary brain tumor in adults with a median survival of approximately 15 months, despite treatment, with most patients experiencing recurrence within 9 months of resection. The propensity of recurrence in GBM exemplifies the fatal course of the disease and remains an underlying area of study as novel instances of recurrence are encountered. The authors present a unique case of a 31-year-old male patient with a history of cerebellomedullary junction astrocytoma who later developed a supratentorial GBM followed by recurrence centered around a preexisting ventriculoperitoneal catheter and located in the hemisphere contralateral to his first GBM. Each of these lesions was initially thought to represent de novo glial neoplasms because of the absence of intervening T2 fluid-attenuated inversion recovery signal change between each lesion. However, next-generation sequencing using the GlioSeq™ platform revealed similar mutational profiles in both GBMs, suggesting an alternative method of migration of tumor cells to the shunt catheter site, and a local inflammatory environment likely triggering recurrence. This study concludes that in rare instances, in the presence of dormant glioma cells, intracranial foreign bodies may promote an inflammatory microenvironment that may activate tumorigenesis.
Figure
Reference
-
1. Batash R, Asna N, Schaffer P, Francis N, Schaffer M. Glioblastoma multiforme, diagnosis and treatment; recent literature review. Curr Med Chem. 2017; 24:3002–3009. PMID: 28521700.2. Alifieris C, Trafalis DT. Glioblastoma multiforme: pathogenesis and treatment. Pharmacol Ther. 2015; 152:63–82. PMID: 25944528.3. Campos B, Olsen LR, Urup T, Poulsen HS. A comprehensive profile of recurrent glioblastoma. Oncogene. 2016; 35:5819–5825. PMID: 27041580.4. Giese A, Kluwe L, Laube B, Meissner H, Berens ME, Westphal M. Migration of human glioma cells on myelin. Neurosurgery. 1996; 38:755–764. PMID: 8692396.5. Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro Oncol. 2013; 15:4–27. PMID: 23136223.6. Kim J, Lee IH, Cho HJ, Park CK, Jung YS, Kim Y, et al. Spatiotemporal evolution of the primary glioblastoma genome. Cancer Cell. 2015; 28:318–328. PMID: 26373279.7. Mickevicius NJ, Carle AB, Bluemel T, Santarriaga S, Schloemer F, Shumate D, et al. Location of brain tumor intersecting white matter tracts predicts patient prognosis. J Neurooncol. 2015; 125:393–400. PMID: 26376654.8. DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012; 246:379–400. PMID: 22435567.9. Galvão RP, Zong H. Inflammation and gliomagenesis: bi-directional communication at early and late stages of tumor progression. Curr Pathobiol Rep. 2013; 1:19–28. PMID: 23538742.10. Ha ET, Antonios JP, Soto H, Prins RM, Yang I, Kasahara N, et al. Chronic inflammation drives glioma growth: cellular and molecular factors responsible for an immunosuppressive microenvironment. Neuroimmunol Neuroinflamm. 2014; 1:66–76.11. Amarante M, Entezami P, Umrau K, Yamamoto J. Ventriculoperitoneal shunt catheter tract glioblastoma multiform concomitant to infection. Interdiscip Neurosurg. 2019; 15:27–29.12. Roldán P, Najarro R, Di Somma A, Culebras D, Hoyos JA, Compta Y, et al. Malignant glioma developed on a patient under deep brain stimulation: pitfalls in management. World Neurosurg. 2019; 129:85–89. PMID: 31158542.13. Linos E, Raine T, Alonso A, Michaud D. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst. 2007; 99:1544–1550. PMID: 17925535.14. Declercq H, De Man R, Vanderperre H, De Praetere P, Wilms G. Peritoneal seeding of a cerebral malignant astrocytoma via a ventriculo-peritoneal shunt. J Belge Radiol. 1992; 75:191–193. PMID: 1400149.15. Narayan A, Jallo G, Huisman TA. Extracranial, peritoneal seeding of primary malignant brain tumors through ventriculo-peritoneal shunts in children: case report and review of the literature. Neuroradiol J. 2015; 28:536–539. PMID: 26443300.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Abdominal Cerebrospinal Pseudocyst: a Complication of Ventriculoperitoneal Shunt in a Child
- A case of Corynebacterium xerosis Infection in Cerebrospinal Fluid and Ventriculoperitoneal Shunt
- Shunt Overdrainage Caused by Displacement of the Pressure Control Cam after Pressure Adjustment
- Malignant Ascites after Subduroperitoneal Shunt in a Patient with Leptomeningeal Metastasis
- Clinical Experience on Lumboperitoneal Shunt