J Rhinol.
2024 Jul;31(2):106-113. 10.18787/jr.2024.00021.
Particulate Matter Induces NLRP3 Inflammasome-Mediated Pyroptosis in Human Nasal Epithelial Cells
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
- 2Clinical Research Institute, Daejeon St. Mary’s Hospital, Daejeon, Republic of Korea
- KMID: 2558237
- DOI: http://doi.org/10.18787/jr.2024.00021
Abstract
- Background and Objectives
Air pollution, particularly particulate matter (PM), has a variety of adverse effects on human health. PM is known to induce cell death through various pathways, including pyroptosis. Despite its significance, research on PM-induced pyroptosis in nasal epithelial cells remains limited. This study aimed to explore PM-induced pyroptosis in cultured human nasal epithelial cells.
Methods
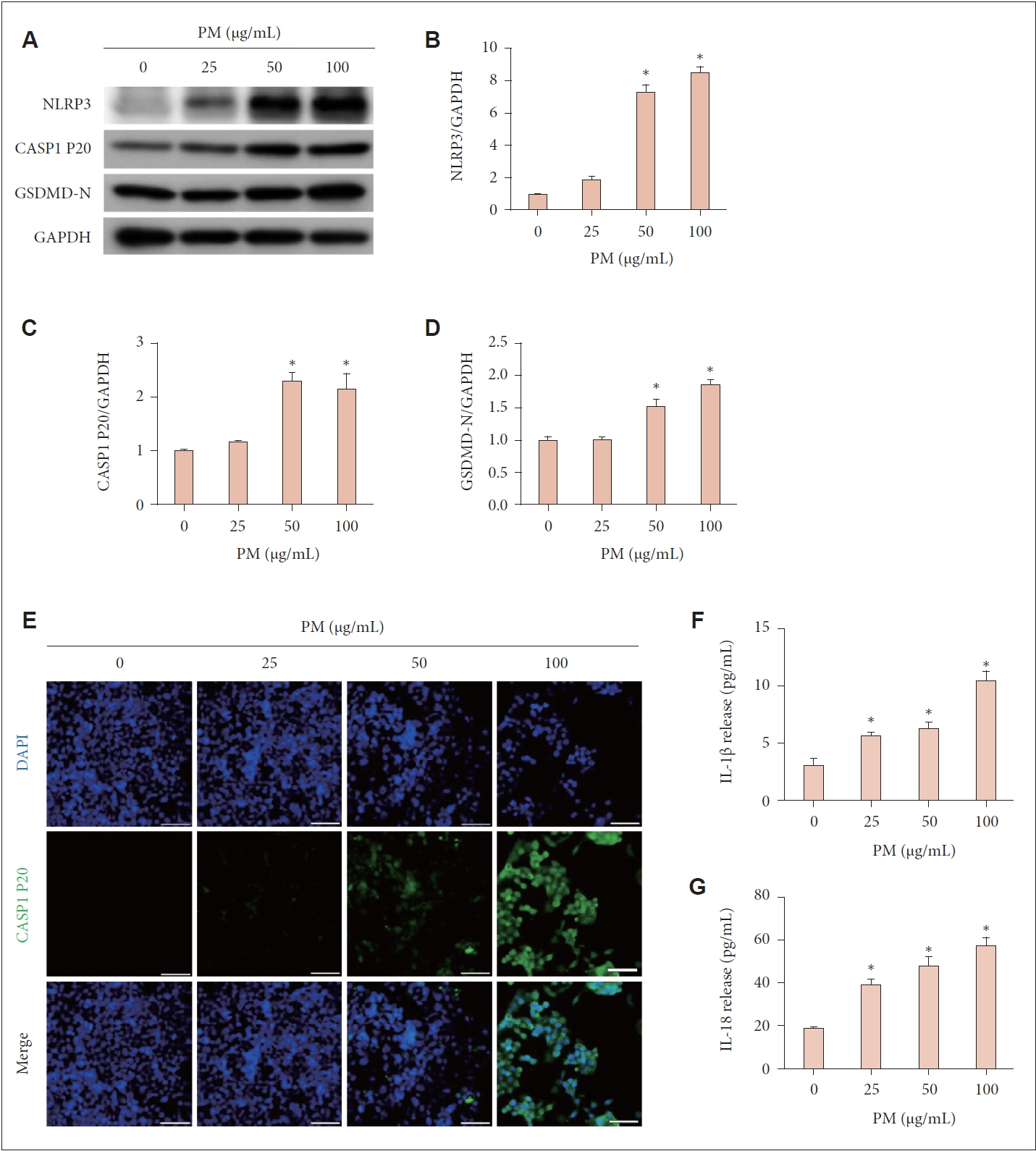
For the in vitro experiments, human nasal epithelial cells were cultured. Cell viability was assessed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, while cell death was evaluated through propidium iodide (PI) staining and lactate dehydrogenase (LDH) release measurement. Protein expression levels related to pyroptosis were examined via western blot using antibodies against NOD-like receptor family, pyrin domain containing 3 (NLRP3), cleaved caspase-1 (CASP1 P20), gasdermin D (GSDMD)-N, and glyceraldehyde phosphate dehydrogenase. Immunofluorescent staining with a CASP1 P20 antibody was conducted to visualize cellular localization. Enzyme-linked immunosorbent assay was utilized to quantify interleukin (IL)-1β and IL-18 protein levels.
Results
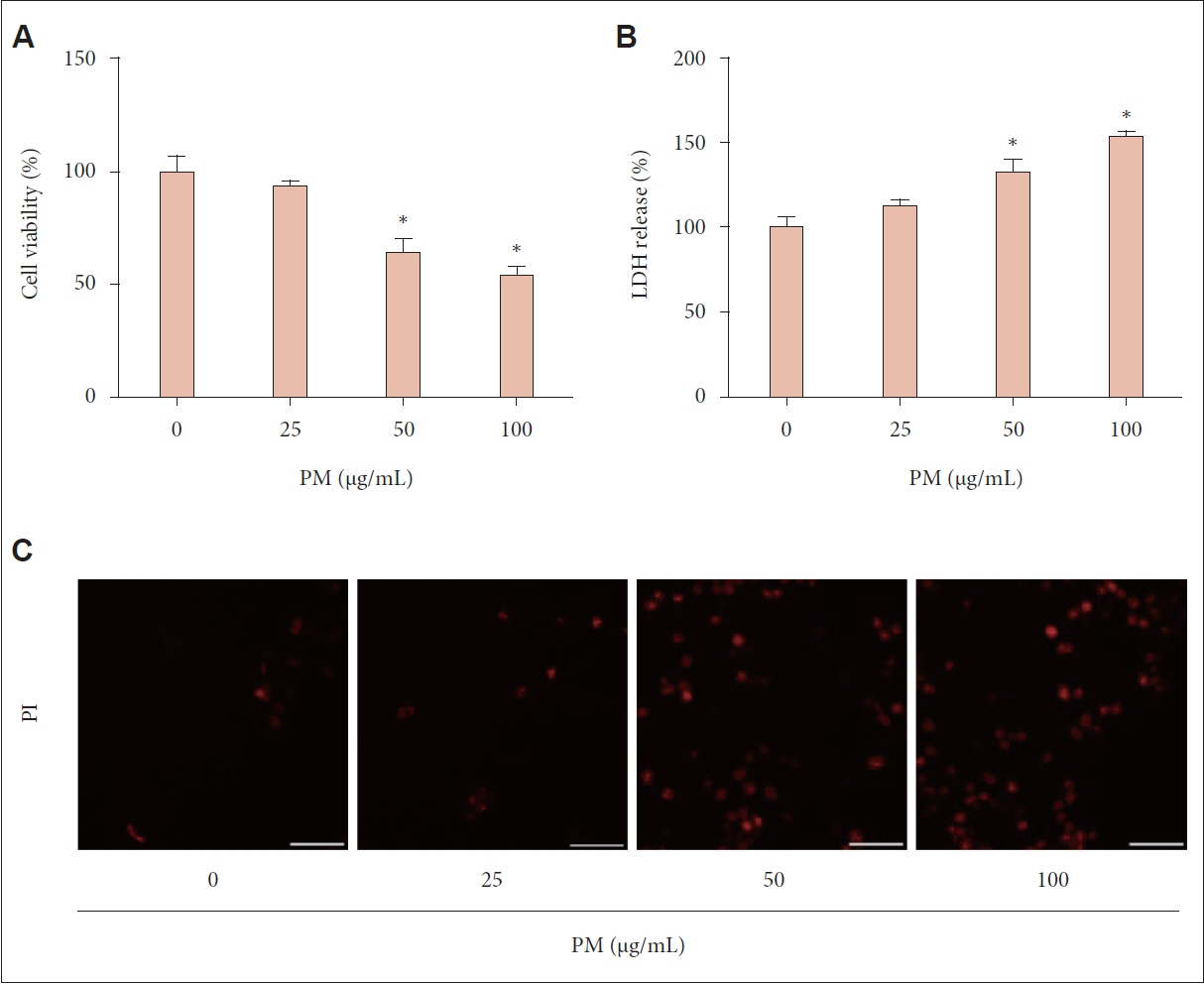
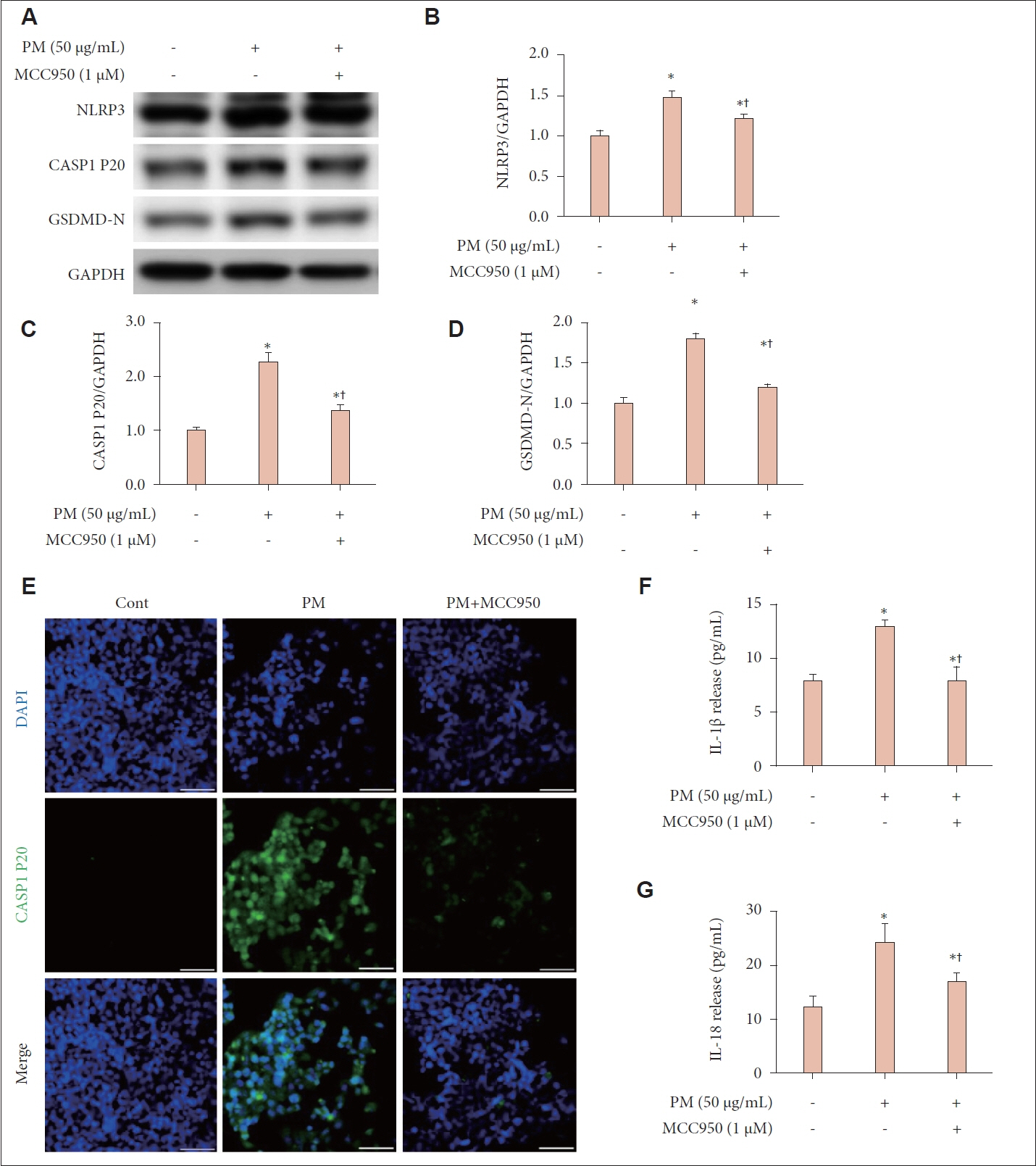
Treatment with PM resulted in decreased cell viability, elevated LDH release, and intensified PI staining, indicating cell death. Pyroptosis was confirmed by the elevated expression of NLRP3, CASP1 P20, and GSDMD-N, along with increased levels of IL-1β and IL-18. Inhibiting the NLRP3 inflammasome with MCC950 reduced the PM-induced effects on protein expression and cytokine release, highlighting the role of the NLRP3 inflammasome in PM-triggered pyroptosis in human nasal epithelial cells.
Conclusion
We showed that PM triggers pyroptosis in human nasal epithelial cells, driven by NLRP3 inflammasome-dependent signaling pathways.
Keyword
Figure
Reference
-
References
1. Atkinson RW, Fuller GW, Anderson HR, Harrison RM, Armstrong B. Urban ambient particle metrics and health: a time-series analysis. Epidemiology. 2010; 21(4):501–11.2. Hasheminassab S, Daher N, Schauer JJ, Sioutas C. Source apportionment and organic compound characterization of ambient ultrafine particulate matter (PM) in the Los Angeles Basin. Atmos Environ. 2013; 79:529–39.
Article3. Kim KH, Kabir E, Kabir S. A review on the human health impact of airborne particulate matter. Environ Int. 2015; 74:136–43.
Article4. Löndahl J, Pagels J, Swietlicki E, Zhou J, Ketzel M, Massling A, et al. A set-up for field studies of respiratory tract deposition of fine and ultrafine particles in humans. J Aerosol Sci. 2006; 37(9):1152–63.
Article5. Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008; 26(4):339–62.6. McGuinn LA, Schneider A, McGarrah RW, Ward-Caviness C, Neas LM, Di Q, et al. Association of long-term PM2.5 exposure with traditional and novel lipid measures related to cardiovascular disease risk. Environ Int. 2019; 122:193–200.7. Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010; 121(21):2331–78.
Article8. Lin H, Qian ZM, Guo Y, Zheng Y, Ai S, Hang J, et al. The attributable risk of chronic obstructive pulmonary disease due to ambient fine particulate pollution among older adults. Environ Int. 2018; 113:143–8.
Article9. Wang BR, Shi JQ, Ge NN, Ou Z, Tian YY, Jiang T, et al. PM2.5 exposure aggravates oligomeric amyloid beta-induced neuronal injury and promotes NLRP3 inflammasome activation in an in vitro model of Alzheimer’s disease. J Neuroinflammation. 2018; 15(1):132.
Article10. Chen S, Zhang Y, Wang Y, Lawrence WR, Rhee J, Guo T, et al. Longterm particulate matter exposure and the risk of neurological hospitalization: evidence from causal inference of a large longitudinal cohort in South China. Chemosphere. 2023; 345:140397.11. Wang L, Luo D, Liu X, Zhu J, Wang F, Li B, et al. Effects of PM2. 5 exposure on reproductive system and its mechanisms. Chemosphere. 2021; 264(Pt 1):128436.12. Risom L, Møller P, Loft S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res. 2005; 592(1-2):119–37.
Article13. Deng X, Zhang F, Rui W, Long F, Wang L, Feng Z, et al. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol In Vitro. 2013; 27(6):1762–70.14. Lee DC, Choi H, Oh JM, Lee DH, Kim SW, Kim SW, et al. Protective effects of α-lipoic acid on cultured human nasal fibroblasts exposed to urban particulate matter. Int Forum Allergy Rhinol. 2019; 9(6):638–47.
Article15. Jin X, Xue B, Zhou Q, Su R, Li Z. Mitochondrial damage mediated by ROS incurs bronchial epithelial cell apoptosis upon ambient PM2.5 exposure. J Toxicol Sci. 2018; 43(2):101–11.16. Wang Y, Zhong Y, Liao J, Wang G. PM2.5-related cell death patterns. Int J Med Sci. 2021; 18(4):1024–9.17. Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019; 29(5):347–64.
Article18. Chen Y, Ye X, Escames G, Lei W, Zhang X, Li M, et al. The NLRP3 inflammasome: contributions to inflammation-related diseases. Cell Mol Biol Lett. 2023; 28(1):51.19. Jia R, Wei M, Lei J, Meng X, Du R, Yang M, et al. PM2.5 induce myocardial injury in hyperlipidemic mice through ROS-pyroptosis signaling pathway. Ecotoxicol Environ Saf. 2023; 254:114699.
Article20. Chen W, Luo Y, Quan J, Zhou J, Yi B, Huang Z. PM2.5 induces renal tubular injury by activating NLRP3-mediated pyroptosis. Ecotoxicol Environ Saf. 2023; 265:115490.
Article21. Niu L, Li L, Xing C, Luo B, Hu C, Song M, et al. Airborne particulate matter (PM2.5) triggers cornea inflammation and pyroptosis via NLRP3 activation. Ecotoxicol Environ Saf. 2021; 207:111306.22. Li J, An Z, Song J, Du J, Zhang L, Jiang J, et al. Fine particulate matterinduced lung inflammation is mediated by pyroptosis in mice. Ecotoxicol Environ Saf. 2021; 219:112351.23. Wang Y, Tang N, Mao M, Zhou Y, Wu Y, Li J, et al. Fine particulate matter (PM2.5) promotes IgE-mediated mast cell activation through ROS/Gadd45b/JNK axis. J Dermatol Sci. 2021; 102(1):47–57.24. Roseborough AD, Zhu Y, Zhao L, Laviolette SR, Pasternak SH, Whitehead SN. Fibrinogen primes the microglial NLRP3 inflammasome and propagates pro-inflammatory signaling via extracellular vesicles: implications for blood-brain barrier dysfunction. Neurobiol Dis. 2023; 177:106001.25. Ren F, Xu J, Zhang J, Xu X, Huang L, Sun W, et al. PM2.5 induced lung injury through upregulating ROS-dependent NLRP3 inflammasome-mediated pyroptosis. Immunobiology. 2022; 227(3):152207.
Article26. Zheng R, Tao L, Jian H, Chang Y, Cheng Y, Feng Y, et al. NLRP3 inflammasome activation and lung fibrosis caused by airborne fine particulate matter. Ecotoxicol Environ Saf. 2018; 163:612–9.27. Li J, Zhang Y, Zhang L, An Z, Song J, Wang C, et al. Fine particulate matter exposure exacerbated nasal mucosal damage in allergic rhinitis mice via NLRP3 mediated pyroptosis. Ecotoxicol Environ Saf. 2021; 228:112998.28. Li Y, Chang LH, Huang WQ, Bao HW, Li X, Chen XH, et al. IL-17A mediates pyroptosis via the ERK pathway and contributes to steroid resistance in CRSwNP. J Allergy Clin Immunol. 2022; 150(2):337–51.29. Schantz MM, Cleveland D, Heckert NA, Kucklick JR, Leigh SD, Long SE, et al. Development of two fine particulate matter standard reference materials (<4 μm and <10 μm) for the determination of organic and inorganic constituents. Anal Bioanal Chem. 2016; 408(16):4257–66.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Particulate Matter on the NLRP3 Inflammasomes in Ocular Tissues and Cervical Lymph Nodes
- Type I Interferon Increases Inflammasomes Associated Pyroptosis in the Salivary Glands of Patients with Primary Sjögren's Syndrome
- The Mechanism of the NLRP3 Inflammasome Activation and Pathogenic Implication in the Pathogenesis of Gout
- Carvacrol attenuated haloperidol-induced Parkinson’s disease via TNF/NFκβ-NLRP3-mediated pyroptosis
- The role of discoid domain receptor 1 on renal tubular epithelial pyroptosis in diabetic nephropathy