Clin Endosc.
2024 Jul;57(4):534-541. 10.5946/ce.2023.164.
Costs involved in compliance with new endoscope reprocessing guidelines
- Affiliations
-
- 1Health Economics Outcomes Research and Market Access, Ambu USA, Columbia, MD, USA
- KMID: 2558111
- DOI: http://doi.org/10.5946/ce.2023.164
Abstract
- Background/Aims
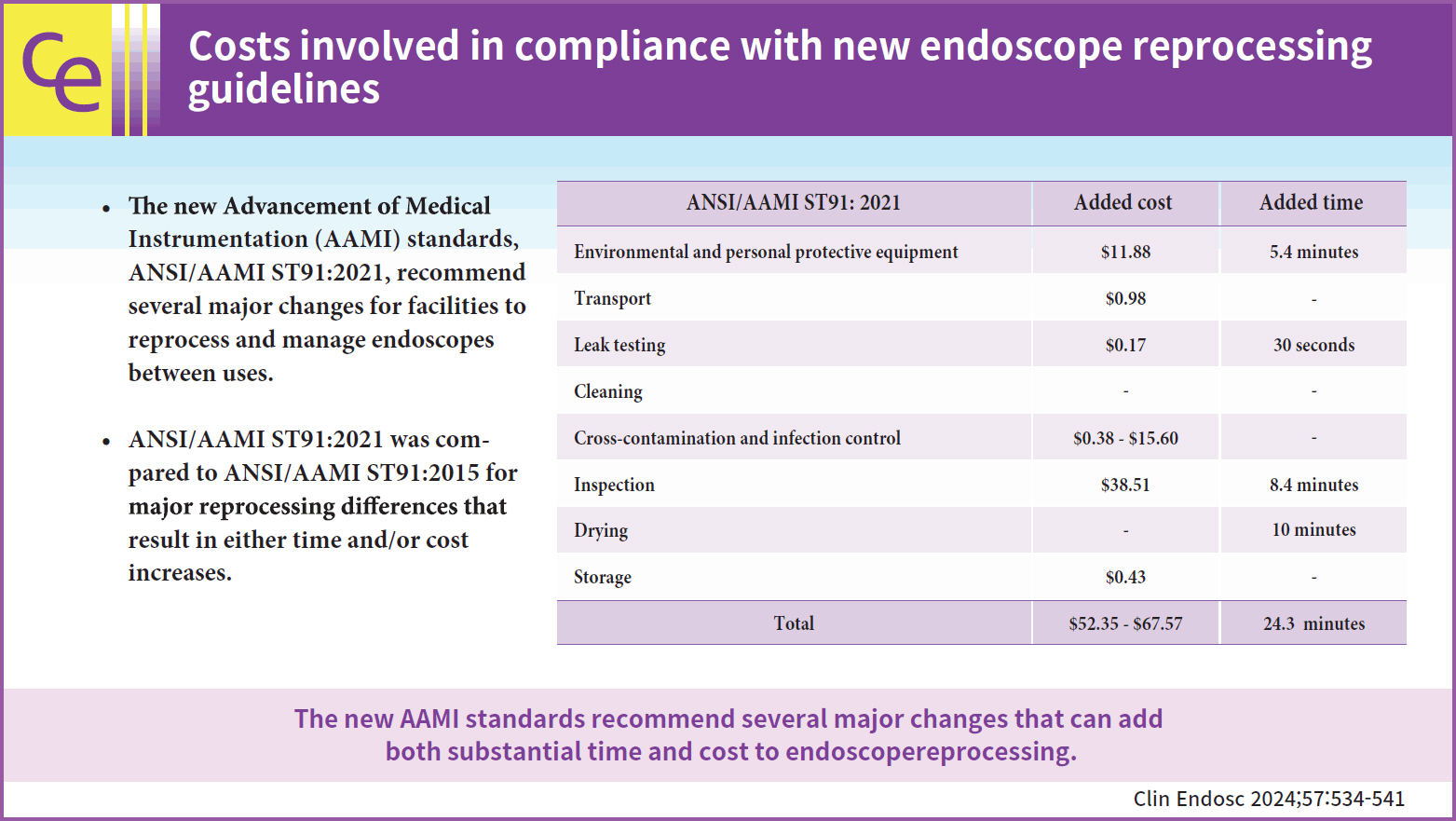
In March 2022, the Association for the Advancement of Medical Instrumentation (AAMI) released the American National Standards Institute (ANSI)/AAMI ST91:2021, their latest update on comprehensive, flexible, and semirigid endoscope reprocessing. These updated standards recommend the sterilization of high-risk endoscopes when possible and provide new recommendations for the precleaning, leak testing, manual cleaning, visual inspection, automated reprocessing, drying, storage, and transport of endoscopes.
Methods
ANSI/AAMI ST91:2021 was compared with ANSI/AAMI ST91:2015 for major reprocessing differences that result in either time and/or cost increases. Time estimates were captured by explicit recommendation inclusion or taken from the literature. All the costs were estimated using publicly available resources.
Results
The updated standards represent a potential 24.3-minute and 52.35 to 67.57 United States dollars increase per procedure in terms of reprocessing time and spending, respectively, not including capital investments. Capital costs per procedure were highly dependent on the procedure volume of the facility.
Conclusions
The new AAMI standards recommend several major changes, such as sterilization, for facilities to reprocess and manage endoscopes between uses. As more facilities increase their reprocessing methods to reflect the updated standards, they do so at a cost and introduce several delays. As the reprocessing landscape evolves, facilities should consider their true costs and alternative solutions, such as single-use endoscopes.
Keyword
Figure
Reference
-
1. Spaner SJ, Warnock GL. A brief history of endoscopy, laparoscopy, and laparoscopic surgery. J Laparoendosc Adv Surg Tech A. 1997; 7:369–373.
Article2. U.S. Food and Drug Administration (FDA). MAUDE: Manufacturer and User Facility Device Experience [Internet]. FDA;2022. [cited 2022 Nov 14]. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm.3. Garrett J. Sterile, single-use bronchoscope reduces associated readmission rates and infection risk: a retrospective clinical analysis. Chest. 2021; 160:A516.
Article4. Mouritsen JM, Ehlers L, Kovaleva J, et al. A systematic review and cost effectiveness analysis of reusable vs. single-use flexible bronchoscopes. Anaesthesia. 2020; 75:529–540.
Article5. Joint Commission Perspectives. Full-year 2019 top standards noncompliance data. Joint Commission Perspectives;2020.6. U.S. Food and Drug Administration (FDA). Factors affecting quality of reprocessing [Internet]. FDA;2018. [cited 2022 Nov 14]. Available from: https://www.fda.gov/medical-devices/reprocessing-reusable-medical-devices/factors-affecting-quality-reprocessing.7. U.S. Food and Drug Administration (FDA). Flexible bronchoscopes and updated recommendations for reprocessing: FDA safety communication [Internet]. FDA;2021. [cited 2022 Nov 14]. Available from: https://www.fda.gov/medical-devices/safety-communications/flexible-bronchoscopes-and-updated-recommendations-reprocessing-fda-safety-communication.8. U.S. Food and Drug Administration (FDA). Update: change in reprocessing methods with certain Karl Storz urological endoscopes-letter to health care providers [Internet]. FDA;2022. [cited 2022 Nov 14]. Available from: https://www.fda.gov/medical-devices/letters-health-care-providers/update-change-reprocessing-methods-certain-karl-storz-urological-endoscopes-letter-health-care.9. Bomman S, Kozarek RA, Thaker AM, et al. Economic burden of enhanced practices of duodenoscopes reprocessing and surveillance: balancing risk and cost containment. Endosc Int Open. 2021; 9:E1404–E1412.
Article10. Association for the Advancement of Medical Instrumentation (AAMI). ANSI/AAMI ST91:2021; Flexible and semi-rigid endoscope processing in health care facilities [Internet]. AAMI;2022. [cited 2022 Nov 14]. Available from: https://www.aami.org/st91.11. United States Bureau of Labor Statistics. Occupational employment and wages, 31-9093 medical equipment preparers [Internet]. United States Bureau of Labor Statistics;2021. [cited 2022 Nov 14]. Available from: https://www.bls.gov/oes/current/oes319093.htm.12. Ofstead CL, Quick MR, Eiland JE, et al. A glimpse at the true cost of reprocessing endoscopes [Internet]. Healthcare Sterile Processing Association;2017. [cited 2022 Nov 14]. Available from: https://www.bostonscientific.com/content/dam/bostonscientific/uro-wh/portfolio-group/LithoVue/pdfs/Sterilization-Resource-Handout.pdf.13. Omidbakhsh N, Manohar S, Vu R, et al. Flexible gastrointestinal endoscope processing challenges, current issues and future perspectives. J Hosp Infect. 2021; 110:133–138.
Article14. Montero MC, Helms A, Mikolajczak A, et al. High-level disinfection evaluation in the ambulatory setting. Am J Infect Control. 2023; 51:225–226.
Article15. Larsen S, Russell RV, Ockert LK, et al. Rate and impact of duodenoscope contamination: a systematic review and meta-analysis. EClinicalMedicine. 2020; 25:100451.
Article16. Ofstead CL, Smart AG, Hopkins KM, et al. The utility of lighted magnification and borescopes for visual inspection of flexible endoscopes. Am J Infect Control. 2023; 51:2–10.
Article17. U.S. Food and Drug Administration (FDA). MAUDE adverse event report: Olympus Medical Systems Corp. bronchoscope unknown. Report Number 2951238-2022-00340 [Internet]. FDA;2022. [cited 2022 Nov 14]. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=13659571&pc=EOQ.18. Andersen CØ, Travis H, Dehlholm-Lambertsen E, et al. The cost of flexible bronchoscopes: a systematic review and meta-analysis. Pharmacoecon Open. 2022; 6:787–797.
Article19. Das A, Cangelosi MJ, Muthusamy VR. A cost-effectiveness analysis of exalt model D single-use duodenoscope versus current duodenoscope reprocessing methods. Tech Innov Gastrointest Endosc. 2022; 24:16–25.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Update of Gastrointestinal Endoscope Reprocessing
- Clinical Practice Guidelines for Endoscope Reprocessing
- Endoscope Reprocessing: Update on Controversial Issues
- Korean Society of Gastrointestinal Endoscopy (KSGE) Guidelines for Endoscope Reprocessing
- Guidelines of cleaning and disinfection in gastrointestinal endoscope for clinicians