J Korean Med Assoc.
2018 Feb;61(2):130-138. 10.5124/jkma.2018.61.2.130.
Guidelines of cleaning and disinfection in gastrointestinal endoscope for clinicians
- Affiliations
-
- 1Korean Society of Digestive Endoscopy Practice Committee, Seoul, Korea. kihong111@hanmail.net
- KMID: 2409021
- DOI: http://doi.org/10.5124/jkma.2018.61.2.130
Abstract
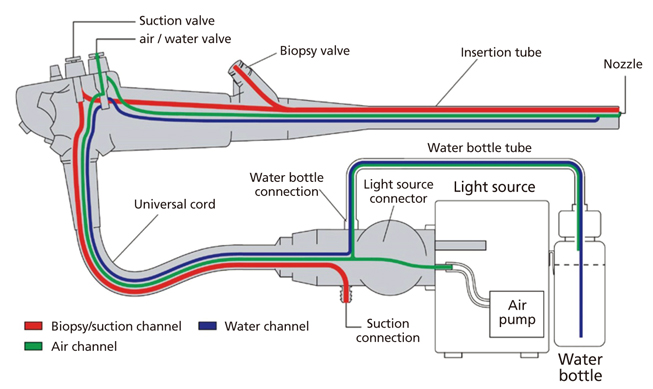
- Gastrointestinal endoscopy plays an important diagnostic and therapeutic role in the field of gastrointestinal disease. As endoscopies have become more common due to the nationwide screening program for digestive cancer and an increasing interest in health among the general public, the risk of infection transmission between patients has emerged as a clinical challenge. Although endoscopes can become highly contaminated with secretions and blood during use, the thorough reprocessing of an endoscope before it is reused in subsequent patients can be difficult due to its complicated structure. Although the incidence of endoscopy-associated infections has been reported to be extremely low, compelling evidence suggests that the actual incidence is underestimated. It has been well established that endoscopes reprocessed appropriately, in accordance with standard guidelines, have no risk of infection transmission. Although revised guidelines for endoscope reprocessing were released in Korea in 2015, suboptimal infection prevention practices during endoscope reprocessing have been reported. Under these circumstances, the Korean Society of Digestive Endoscopy developed the "˜Guidelines of cleaning and disinfection in gastrointestinal endoscope for clinicians' based on the currently available evidence. These guidelines provide accurate and updated information on reprocessing techniques, and can help improve the quality of reprocessing and compliance by health care personnel. As a result, infection control during gastrointestinal endoscopies can be expected to be achieved in Korea.
Keyword
MeSH Terms
Figure
Reference
-
1. Kenters N, Huijskens EG, Meier C, Voss A. Infectious diseases linked to cross-contamination of flexible endoscopes. Endosc Int Open. 2015; 3:E259–E265.
Article2. Dirlam Langlay AM, Ofstead CL, Mueller NJ, Tosh PK, Baron TH, Wetzler HP. Reported gastrointestinal endoscope reprocessing lapses: the tip of the iceberg. Am J Infect Control. 2013; 41:1188–1194.
Article3. Nelson DB, Muscarella LF. Current issues in endoscope reprocessing and infection control during gastrointestinal endoscopy. World J Gastroenterol. 2006; 12:3953–3964.
Article4. Korean Society of Gastrointestinal Endoscopy. Guideline for cleaning and disinfecting gastrointestinal endoscopes [Internet]. Seoul: Korean Society of Gastrointestinal Endoscopy;2015. cited 2018 Jan 22. Available from: https://www.gie.or.kr/upload/pds/disinfection_guide_3.pdf.5. Cha JM, Moon JS, Chung IK, Kim JO, Im JP, Cho YK, Kim HG, Lee SK, Lee HL, Jang JY, Kim ES, Jung Y, Moon CM, Kim Y, Park BY. National endoscopy quality improvement program remains suboptimal in Korea. Gut Liver. 2016; 10:699–705.
Article6. ASGE Quality Assurance in Endoscopy Committee. Petersen BT, Chennat J, Cohen J, Cotton PB, Greenwald DA, Kowalski TE, Krinsky ML, Park WG, Pike IM, Romagnuolo J. Society for Healthcare Epidemiology of America, Rutala WA. Multisociety guideline on reprocessing flexible gastrointestinal endoscopes: 2011. Gastrointest Endosc. 2011; 73:1075–1084.
Article7. Nelson DB. Infectious disease complications of GI endoscopy. Part II. Exogenous infections. Gastrointest Endosc. 2003; 57:695–711.
Article8. Spaulding EH. Chemical disinfection and antisepsis in the hospital. J Hosp Res. 1972; 9:5–31.9. Ministry of Health and Welfare. Disinfection guideline for medical instrument [Internet]. Sejong: National Law Information Center;2010. cited 2018 Jan 22. Available from: http://www.law.go.kr/admRulLsInfoP.do?admRulSeq=2000000014476.10. US Food and Drug Administration. Reprocessing medical devices in health care settings: validation methods and labeling [Internet]. Silver Spring: US Food and Drug Administration;2015. cited 2018 Jan 22. Available from: http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm253010.pdf.11. Rey JF, Bjorkman D, Nelson D, Duforest-Rey D, Axon A, Saenz R, Fried M, Mine T, Ogoshi K, Krabshuis J, LeMair A. Endoscope disinfection: a resource-sensitive approach [Internet]. Milwaukee: World Gastroenterology Organization;2011. cited 2018 Jan 22. Available from: http://www.worldgastroenterology.org/UserFiles/file/guidelines/endoscope-disinfection-english-2011.pdf.12. Association for the Advancement of Medical Instrumentation. American National Standard ANSI/AAMI ST91:2015: flexible and semi-rigid endoscope processing in health care facilities. Arlington: Association for the Advancement of Medical Instrumentation;2015.13. Society of Gastroenterology Nurses and Associates. Standards of infection prevention in reprocessing of flexible gastrointestinal endoscopes. Chicago: Society of Gastroenterology Nurses and Associates;2016.14. Beilenhoff U, Neumann CS, Rey JF, Biering H, Blum R, Cimbro M, Kampf B, Rogers M, Schmidt V;. ESGE-ESGENA guideline: cleaning and disinfection in gastrointestinal endoscopy. Endoscopy. 2008; 40:939–957.
Article15. British Society of Gastroenterology Endoscopy Committee. BSG guidance on for decontamination of equipment for gastrointestinal endoscopy [Internet]. London: British Society of Gastroenterology;2014. cited 2018 Jan 22. Available from: https://www.bsg.org.uk/asset/F28EDCE3-11FC-45B7-B204D3034251D6B9/.16. Gastroenterological Society of Australia. Infection control in endoscopy [Internet]. Melbourne: Gastroenterological Society of Australia;2010. cited 2018 Jan 22. Available from: http://www.gesa.org.au/resources/infection-control-in-endoscopy/.17. Schmelzer M, Daniels G, Hough H. Safe storage time for reprocessed flexible endoscopes: a systematic review. JBI Database System Rev Implement Rep. 2015; 13:187–243.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Steps of Reprocessing and Equipments
- Korean Society of Gastrointestinal Endoscopy (KSGE) Guidelines for Endoscope Reprocessing
- Endoscope Reprocessing: Update on Controversial Issues
- Current Issues in Duodenoscope-Associated Infections: Now Is the Time to Take Action
- Adequacy of Reprocessing Gastrointestinal Endoscopes in Korea Hospitals