Diabetes Metab J.
2024 Jul;48(4):752-762. 10.4093/dmj.2023.0305.
Temporal Changes in Resting Heart Rate and Risk of Diabetes Mellitus
- Affiliations
-
- 1Division of Population Health Research, Department of Precision Medicine, Korea National Institute of Health, Cheongju, Korea
- 2Korea National Institute of Health, Cheongju, Korea
- KMID: 2558042
- DOI: http://doi.org/10.4093/dmj.2023.0305
Abstract
- Background
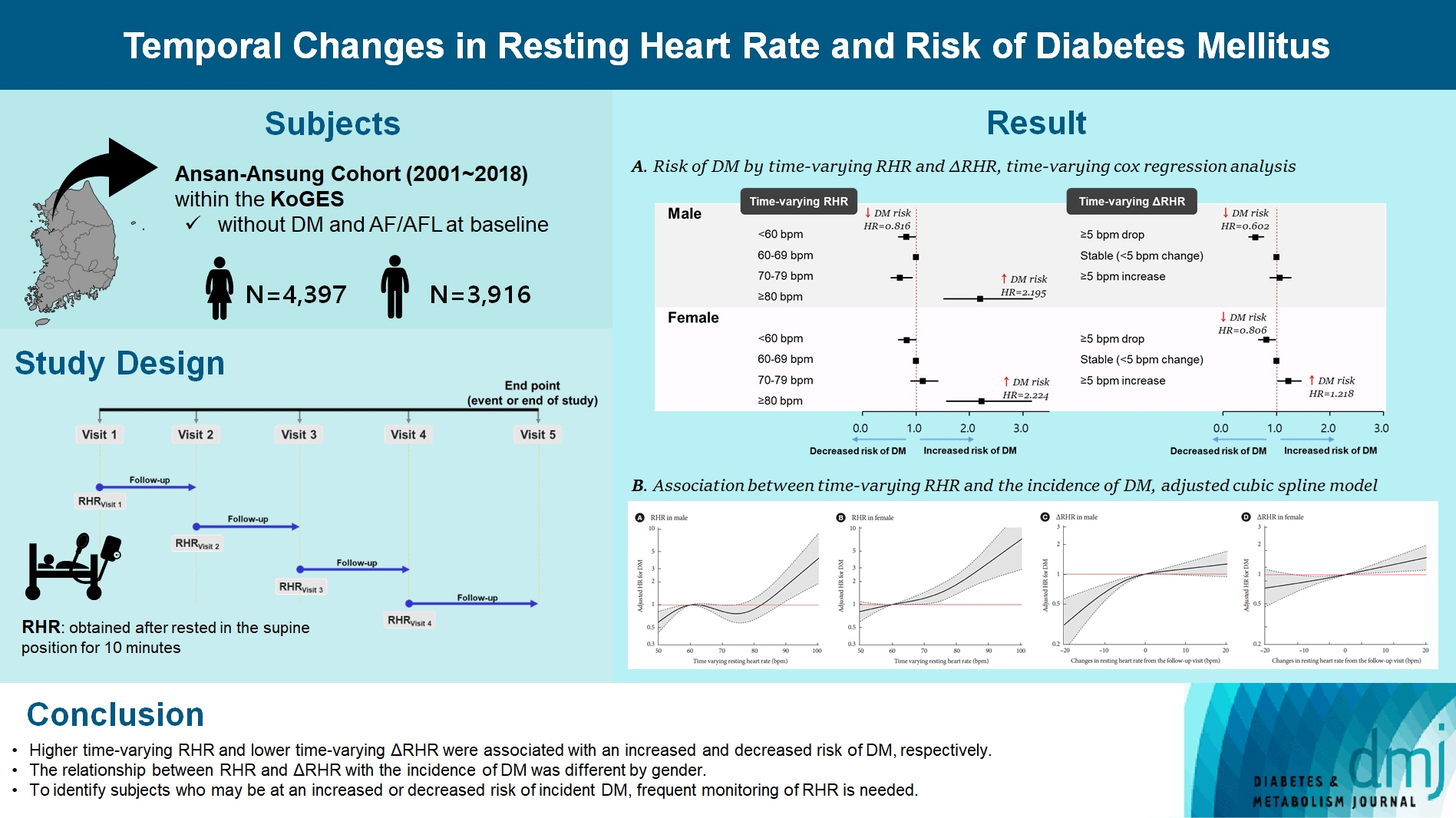
To investigate the association between the time-varying resting heart rate (RHR) and change in RHR (∆RHR) over time and the risk of diabetes mellitus (DM) by sex.
Methods
We assessed 8,392 participants without DM or atrial fibrillation/flutter from the Korean Genome and Epidemiology Study, a community-based prospective cohort study that was initiated in 2001 to 2002. The participants were followed up until December 31, 2018. Updating RHR with biennial in-study re-examinations, the time-varying ∆RHR was calculated by assessing the ∆RHR at the next follow-up visit.
Results
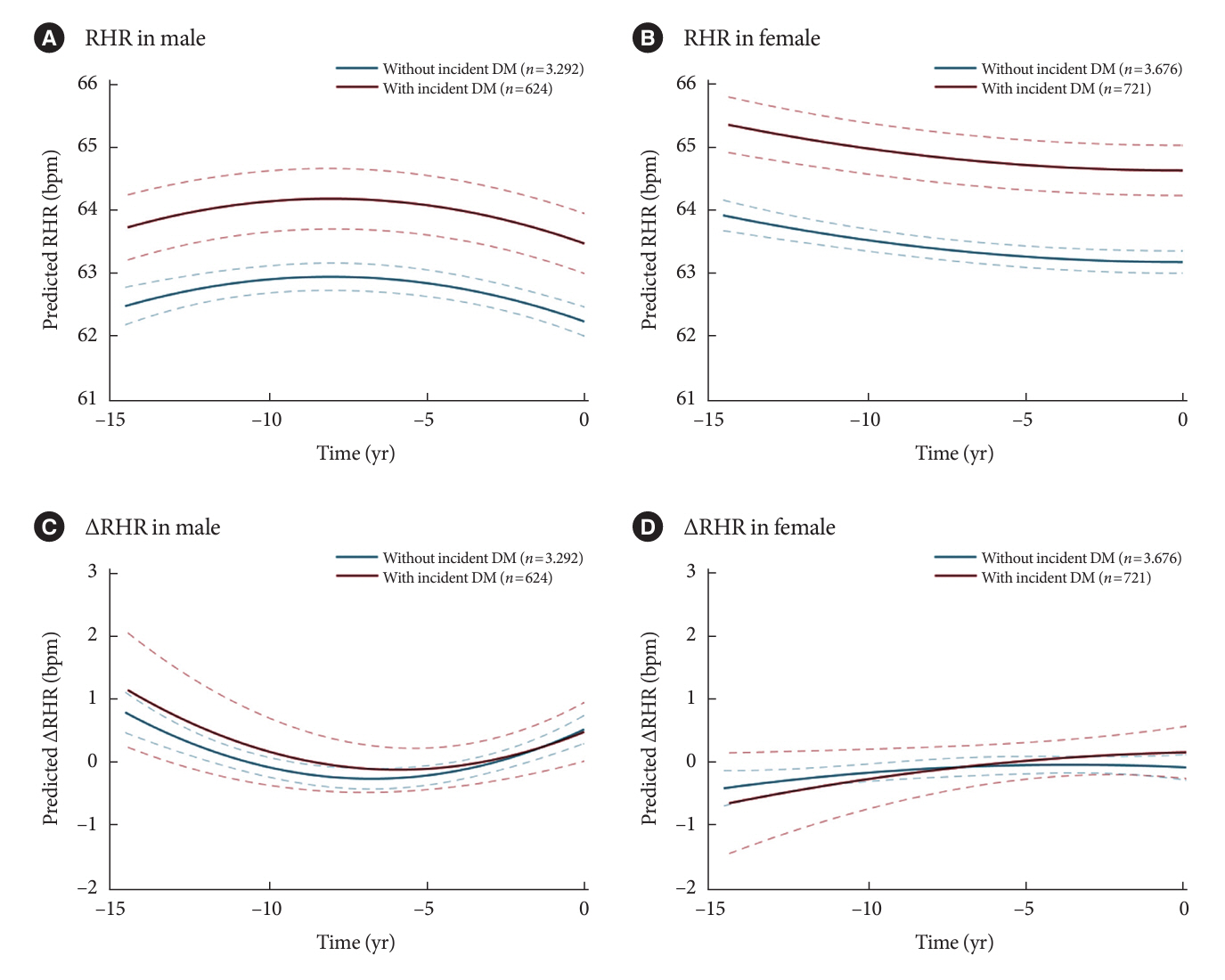
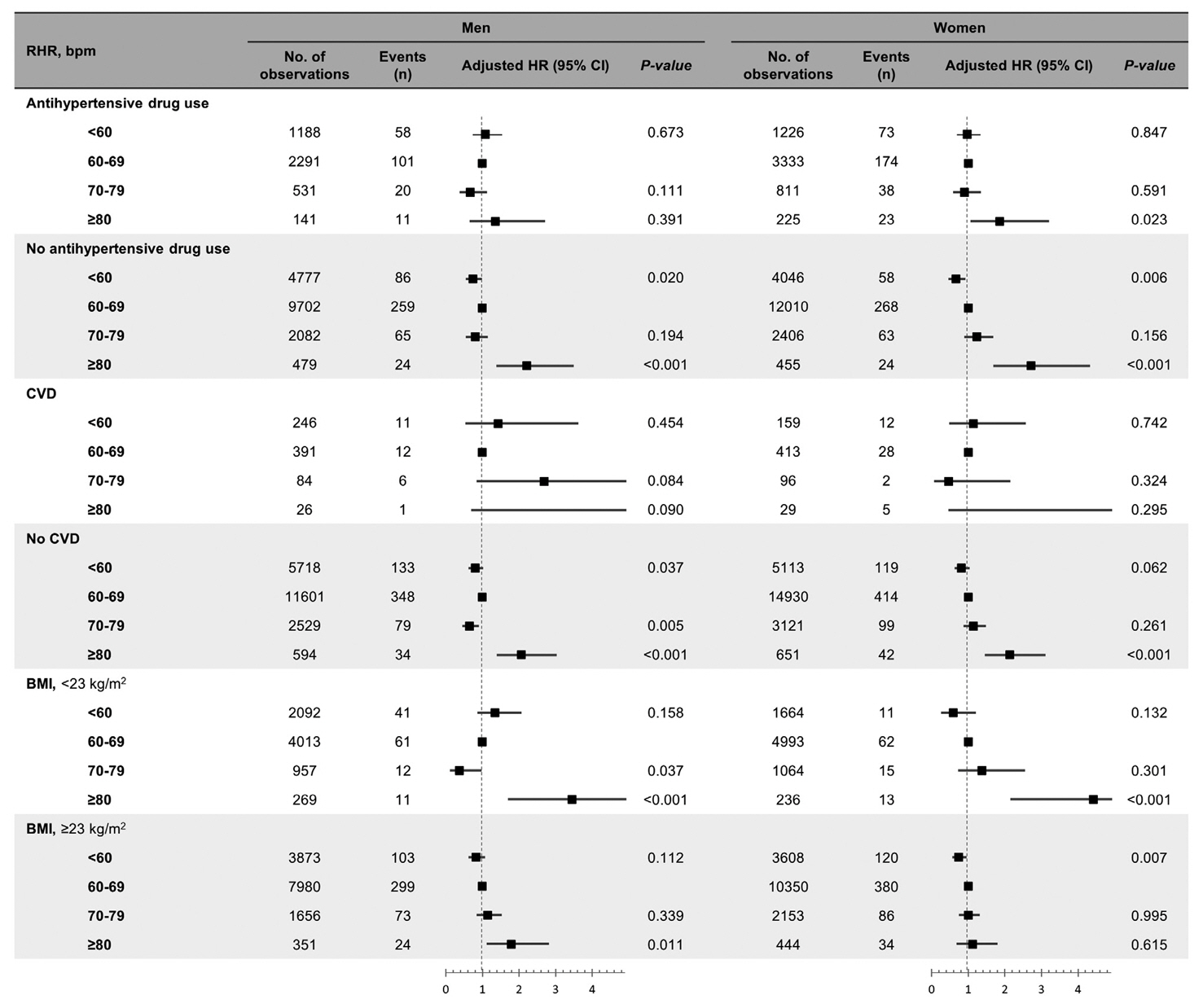
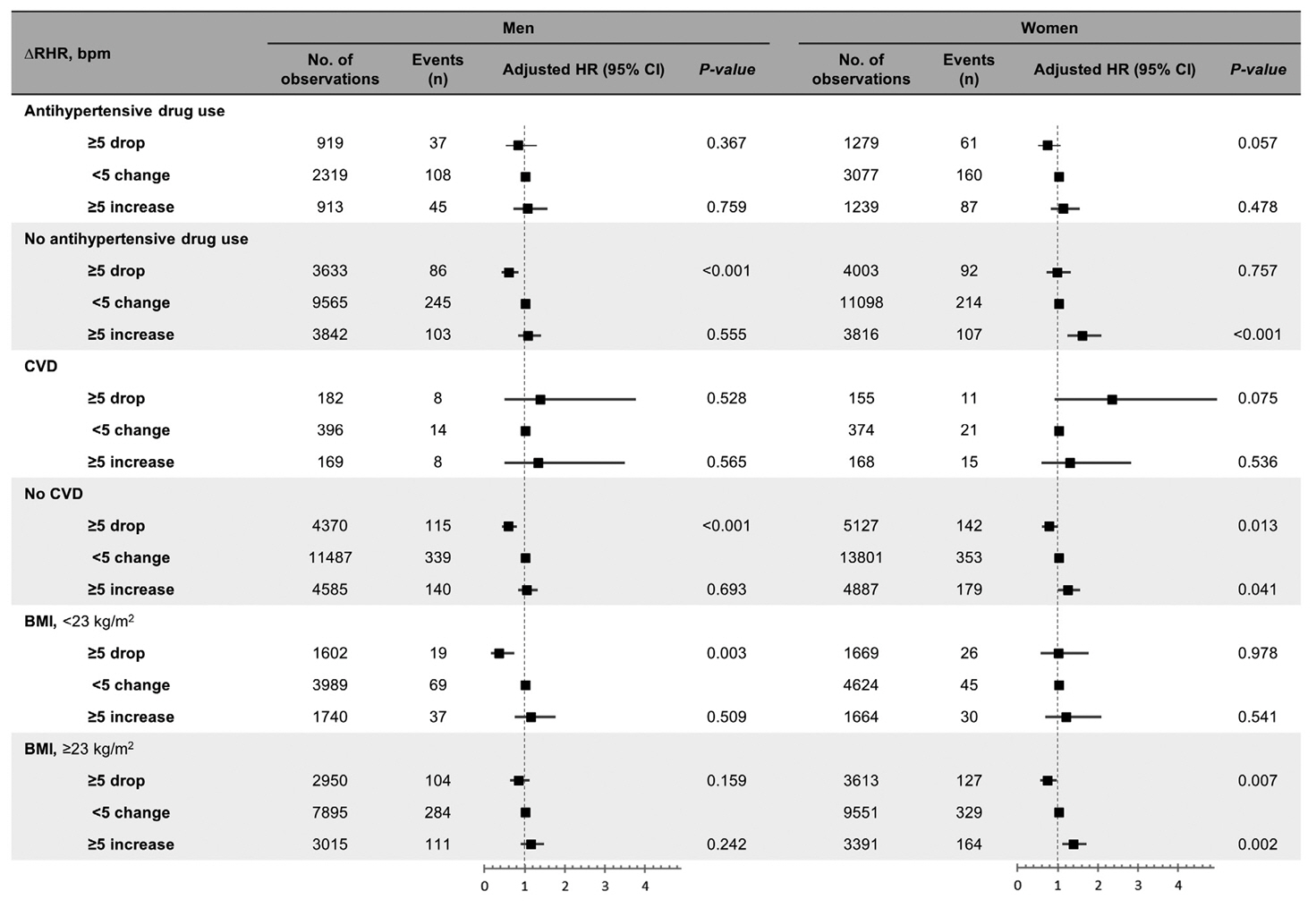
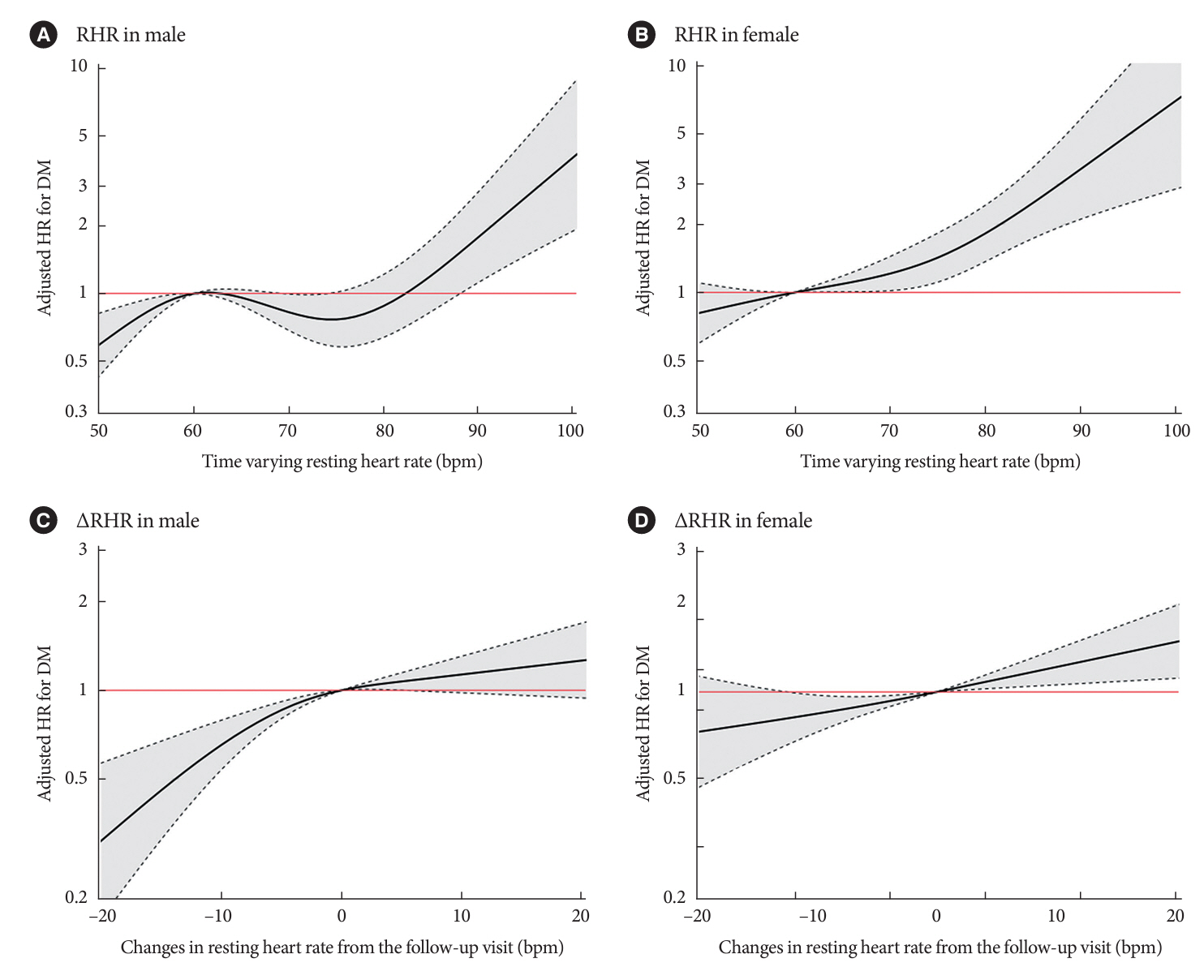
Over a median follow-up of 12.3 years, 1,345 participants (16.2%) had DM. As compared with RHR of 60 to 69 bpm, for RHR of ≥80 bpm, the incidence of DM was significantly increased for both male and female. A drop of ≥5 bpm in ∆RHR when compared with the stable ∆RHR group (–5< ∆RHR <5 bpm) was associated significantly with lower risk of DM in both male and female. However, an increase of ≥5 bpm in ∆RHR was significantly associated with higher risk of DM only in female, not in male (hazard ratio for male, 1.057 [95% confidence interval, 0.869 to 1.285]; and for female, 1.218 [95% confidence interval, 1.008 to 1.471]).
Conclusion
In this community-based longitudinal cohort study, a reduction in ∆RHR was associated with a decreased risk of DM, while an increase in ∆RHR was associated with an increased risk of DM only in female.
Keyword
Figure
Reference
-
1. GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018; 392:1923–94.2. World Health Organization. Global report on diabetes. Available from: https://www.who.int/publications/i/item/9789241565257 (cited 2023 Nov 22).3. International Diabetes Federation. IDF Diabetes Atlas. 9th ed. Brussels: IDF;2019.4. Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011; 94:311–21.5. Sabbah HN, Ilsar I, Zaretsky A, Rastogi S, Wang M, Gupta RC. Vagus nerve stimulation in experimental heart failure. Heart Fail Rev. 2011; 16:171–8.
Article6. Shcherbina A, Mattsson CM, Waggott D, Salisbury H, Christle JW, Hastie T, et al. Accuracy in wrist-worn, sensor-based measurements of heart rate and energy expenditure in a diverse cohort. J Pers Med. 2017; 7:3.
Article7. Aune D, Hartaigh BO, Vatten LJ. Resting heart rate and the risk of type 2 diabetes: a systematic review and dose: response meta-analysis of cohort studies. Nutr Metab Cardiovasc Dis. 2015; 25:526–34.8. Wang L, Cui L, Wang Y, Vaidya A, Chen S, Zhang C, et al. Resting heart rate and the risk of developing impaired fasting glucose and diabetes: the Kailuan prospective study. Int J Epidemiol. 2015; 44:689–99.
Article9. Zhao Y, Zhang M, Liu Y, Yin Z, Li H, Sun H, et al. 6-Year change in resting heart rate is associated with incident type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2019; 29:236–43.10. Nauman J, Janszky I, Vatten LJ, Wisloff U. Temporal changes in resting heart rate and deaths from ischemic heart disease. JAMA. 2011; 306:2579–87.
Article11. Eppinga RN, Hagemeijer Y, Burgess S, Hinds DA, Stefansson K, Gudbjartsson DF, et al. Identification of genomic loci associated with resting heart rate and shared genetic predictors with all-cause mortality. Nat Genet. 2016; 48:1557–63.
Article12. Liu D, Qin P, Liu Y, Sun X, Li H, Wu X, et al. Sex-specific association of resting heart rate with type 2 diabetes mellitus. J Diabetes Complications. 2020; 34:107754.
Article13. Kim Y, Han BG; KoGES group. Cohort profile: the Korean Genome and Epidemiology Study (KoGES) consortium. Int J Epidemiol. 2017; 46:e20.
Article14. Allison PD. Survival analysis using SAS: a practical guide. 2nd ed. Cary: SAS Institute;2010.15. Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999; 20:145–57.
Article16. Vazir A, Claggett B, Pitt B, Anand I, Sweitzer N, Fang J, et al. Prognostic importance of temporal changes in resting heart rate in heart failure and preserved ejection fraction: from the TOPCAT Study. JACC Heart Fail. 2017; 5:782–91.17. Cui X, Mandalenakis Z, Thunstrom E, Fu M, Svardsudd K, Hansson PO. The impact of time-updated resting heart rate on cause-specific mortality in a random middle-aged male population: a lifetime follow-up. Clin Res Cardiol. 2021; 110:822–30.
Article18. Archangelidi O, Pujades-Rodriguez M, Timmis A, Jouven X, Denaxas S, Hemingway H. Clinically recorded heart rate and incidence of 12 coronary, cardiac, cerebrovascular and peripheral arterial diseases in 233,970 men and women: a linked electronic health record study. Eur J Prev Cardiol. 2018; 25:1485–95.
Article19. Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ. 2010; 340:c2289.20. Smabrekke B, Rinde LB, Hindberg K, Hald EM, Vik A, Wilsgaard T, et al. Atherosclerotic risk factors and risk of myocardial infarction and venous thromboembolism; time-fixed versus time-varying analyses: the Tromso Study. PLoS One. 2016; 11:e0163242.21. Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004; 25:363–70.
Article22. Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, et al. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007; 49:27–33.
Article23. Flanagan DE, Vaile JC, Petley GW, Moore VM, Godsland IF, Cockington RA, et al. The autonomic control of heart rate and insulin resistance in young adults. J Clin Endocrinol Metab. 1999; 84:1263–7.
Article24. Mancia G, Bousquet P, Elghozi JL, Esler M, Grassi G, Julius S, et al. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007; 25:909–20.25. Jamerson KA, Julius S, Gudbrandsson T, Andersson O, Brant DO. Reflex sympathetic activation induces acute insulin resistance in the human forearm. Hypertension. 1993; 21:618–23.
Article26. Kim DI, Yang HI, Park JH, Lee MK, Kang DW, Chae JS, et al. The association between resting heart rate and type 2 diabetes and hypertension in Korean adults. Heart. 2016; 102:1757–62.
Article27. Vazir A, Claggett B, Cheng S, Skali H, Shah A, Agulair D, et al. Association of resting heart rate and temporal changes in heart rate with outcomes in participants of the atherosclerosis risk in communities study. JAMA Cardiol. 2018; 3:200–6.
Article28. Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004; 15:2792–800.29. Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998; 31(1 Pt 2):409–14.30. Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997; 100:270–8.
Article31. Ghadge AA, Khaire AA. Leptin as a predictive marker for metabolic syndrome. Cytokine. 2019; 121:154735.
Article32. Shamsuzzaman AS, Winnicki M, Wolk R, Svatikova A, Phillips BG, Davison DE, et al. Independent association between plasma leptin and C-reactive protein in healthy humans. Circulation. 2004; 109:2181–5.
Article33. Cowan MJ, Pike K, Burr RL. Effects of gender and age on heart rate variability in healthy individuals and in persons after sudden cardiac arrest. J Electrocardiol. 1994; 27 Suppl:1–9.
Article34. Dart AM, Du XJ, Kingwell BA. Gender, sex hormones and autonomic nervous control of the cardiovascular system. Cardiovasc Res. 2002; 53:678–87.35. Sato N, Miyake S, Akatsu J, Kumashiro M. Power spectral analysis of heart rate variability in healthy young women during the normal menstrual cycle. Psychosom Med. 1995; 57:331–5.
Article36. Saeki Y, Atogami F, Takahashi K, Yoshizawa T. Reflex control of autonomic function induced by posture change during the menstrual cycle. J Auton Nerv Syst. 1997; 66:69–74.
Article37. Kim SH, Shin DW, Kim S, Han K, Park SH, Kim YH, et al. Prescribing patterns of antihypertensives for treatment-naive patients in South Korea: from Korean NHISS claim data. Int J Hypertens. 2019; 2019:4735876.38. Black A, Murray L, Cardwell C, Smith GD, McCarron P. Secular trends in heart rate in young adults, 1949 to 2004: analyses of cross sectional studies. Heart. 2006; 92:468–73.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association of Resting Heart Rate and Dyslipidemia and Diabetes in 2018 National Health and Nutrition Examination Survey
- Joint Association of Relative Grip Strength and Resting Heart Rate with the Risk of Developing Diabetes in Middle-Aged Adults
- Letter: Effects of High-Dose α-Lipoic Acid on Heart Rate Variability of Type 2 Diabetes Mellitus Patients with Cardiac Autonomic Neuropathy in Korea (Diabetes Metab J 2017;41:275-83)
- Smoking and Type 2 Diabetes Mellitus
- Clinical Significance of Treadmill Exercise Test in Diabetes Mellitus