Diabetes Metab J.
2024 Jul;48(4):716-729. 10.4093/dmj.2023.0031.
Diabetes Promotes Myocardial Fibrosis via AMPK/EZH2/PPAR-γ Signaling Pathway
- Affiliations
-
- 1Department of Cardiology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi Medical Center, Nanjing Medical University, Wuxi, China
- KMID: 2558039
- DOI: http://doi.org/10.4093/dmj.2023.0031
Abstract
- Background
Diabetes-induced cardiac fibrosis is one of the main mechanisms of diabetic cardiomyopathy. As a common histone methyltransferase, enhancer of zeste homolog 2 (EZH2) has been implicated in fibrosis progression in multiple organs. However, the mechanism of EZH2 in diabetic myocardial fibrosis has not been clarified.
Methods
In the current study, rat and mouse diabetic model were established, the left ventricular function of rat and mouse were evaluated by echocardiography and the fibrosis of rat ventricle was evaluated by Masson staining. Primary rat ventricular fibroblasts were cultured and stimulated with high glucose (HG) in vitro. The expression of histone H3 lysine 27 (H3K27) trimethylation, EZH2, and myocardial fibrosis proteins were assayed.
Results
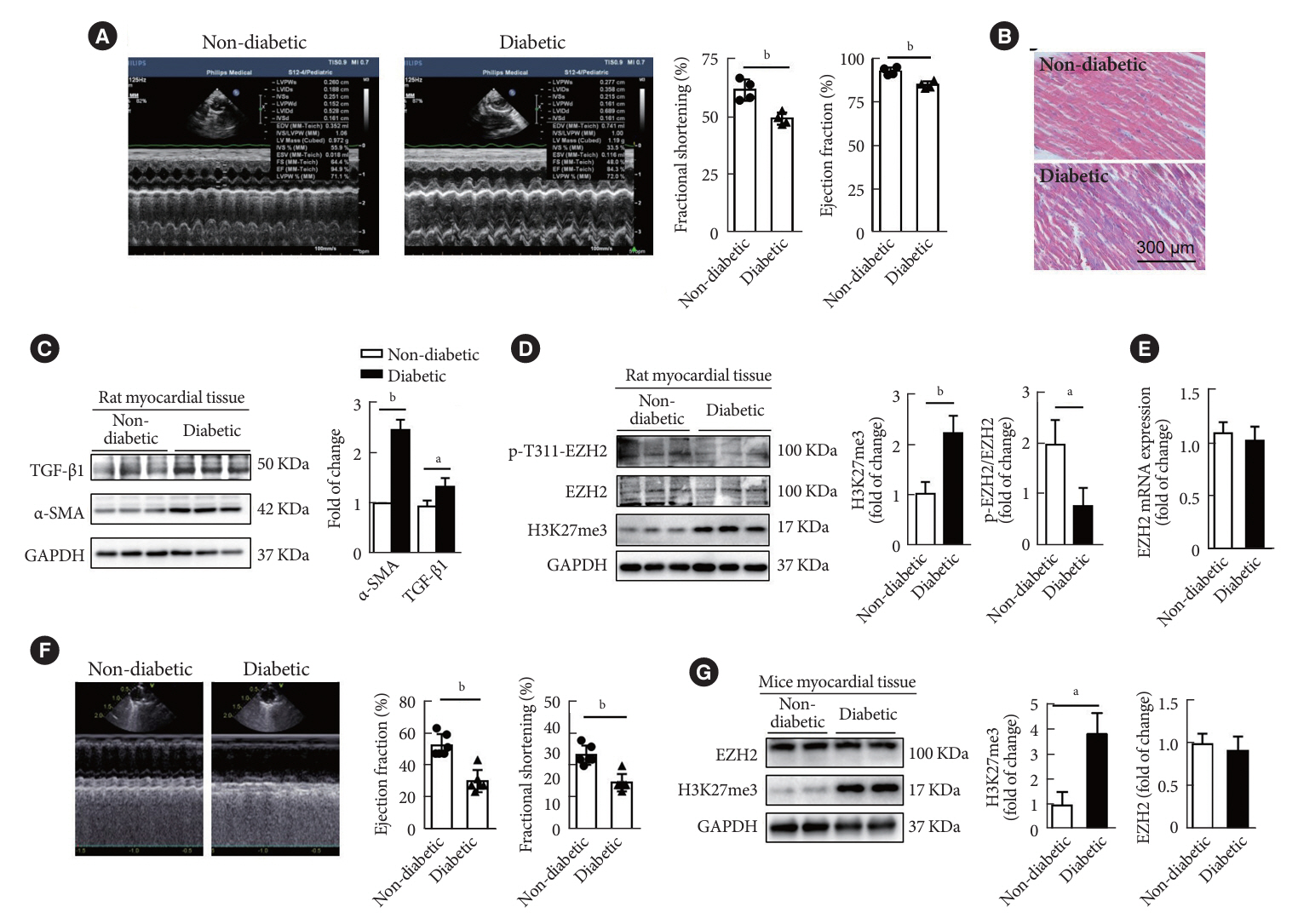
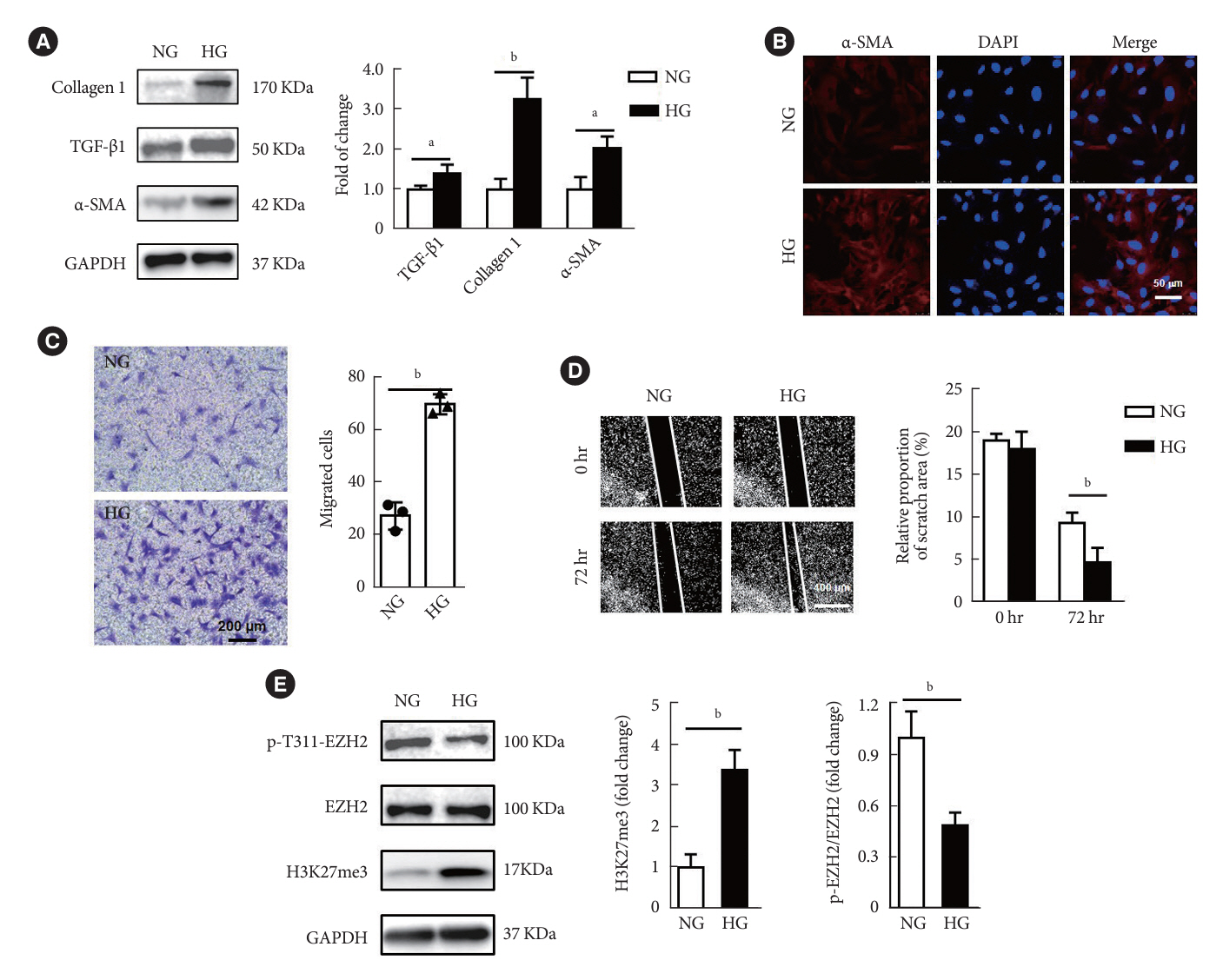
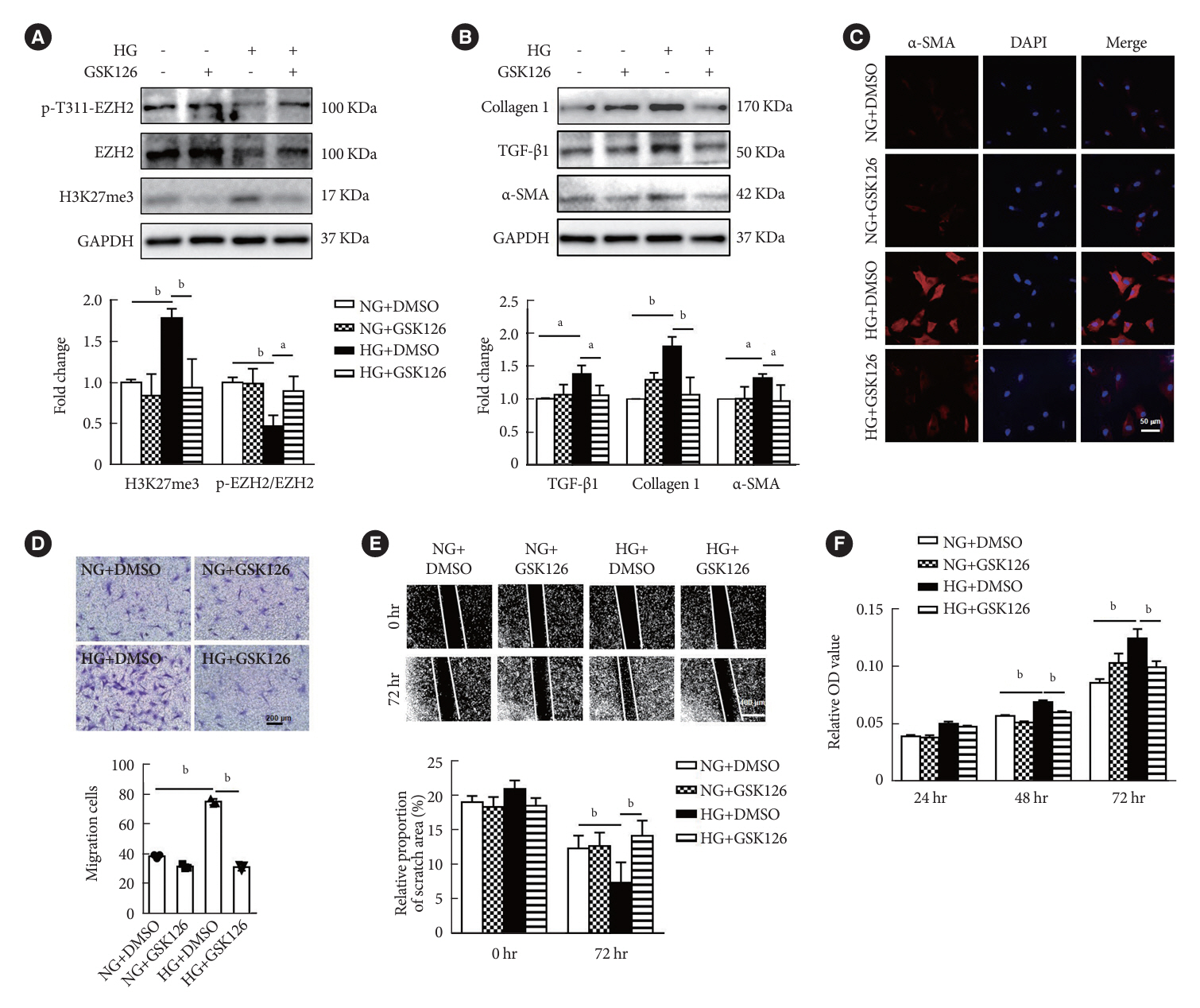
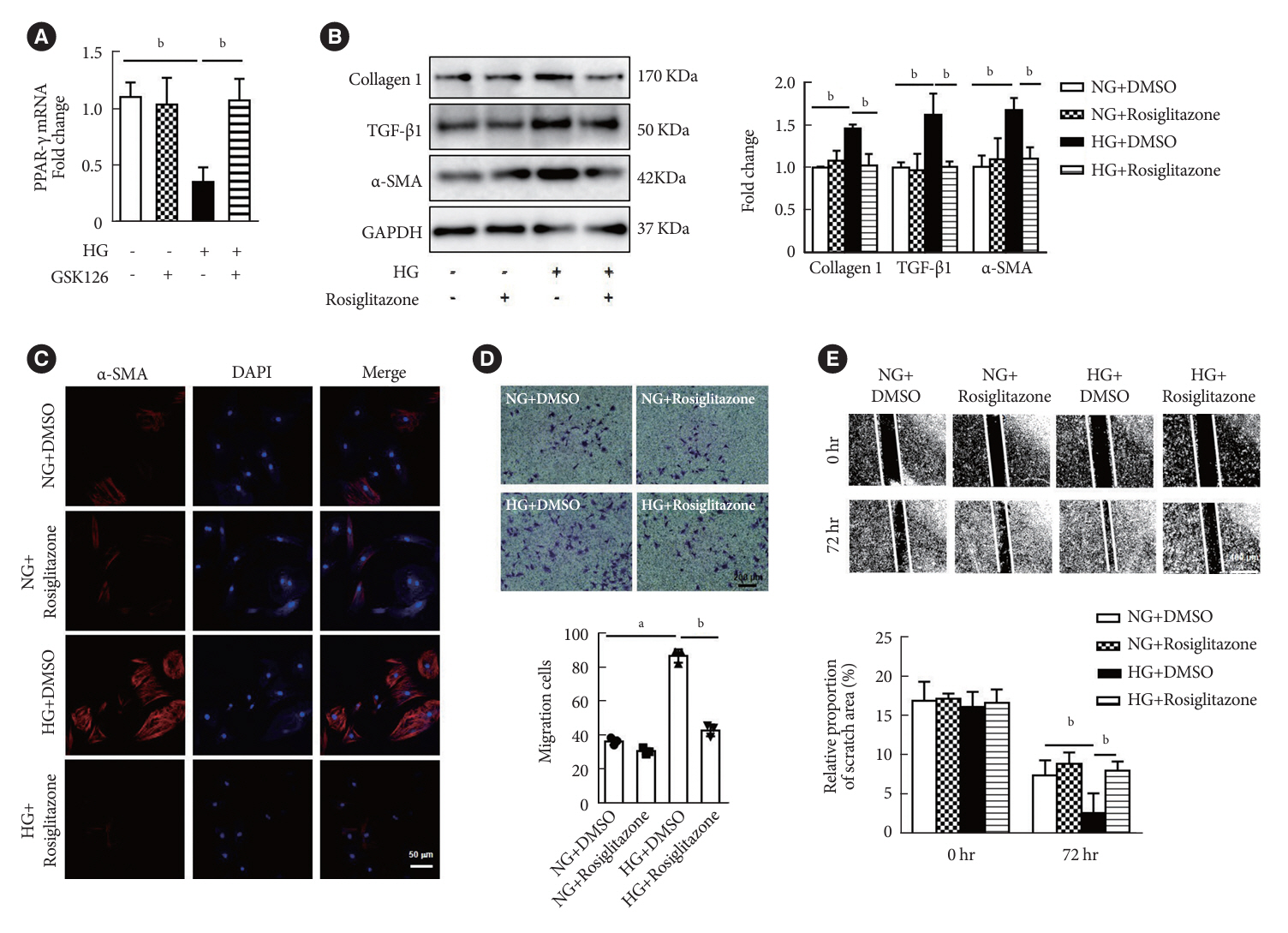
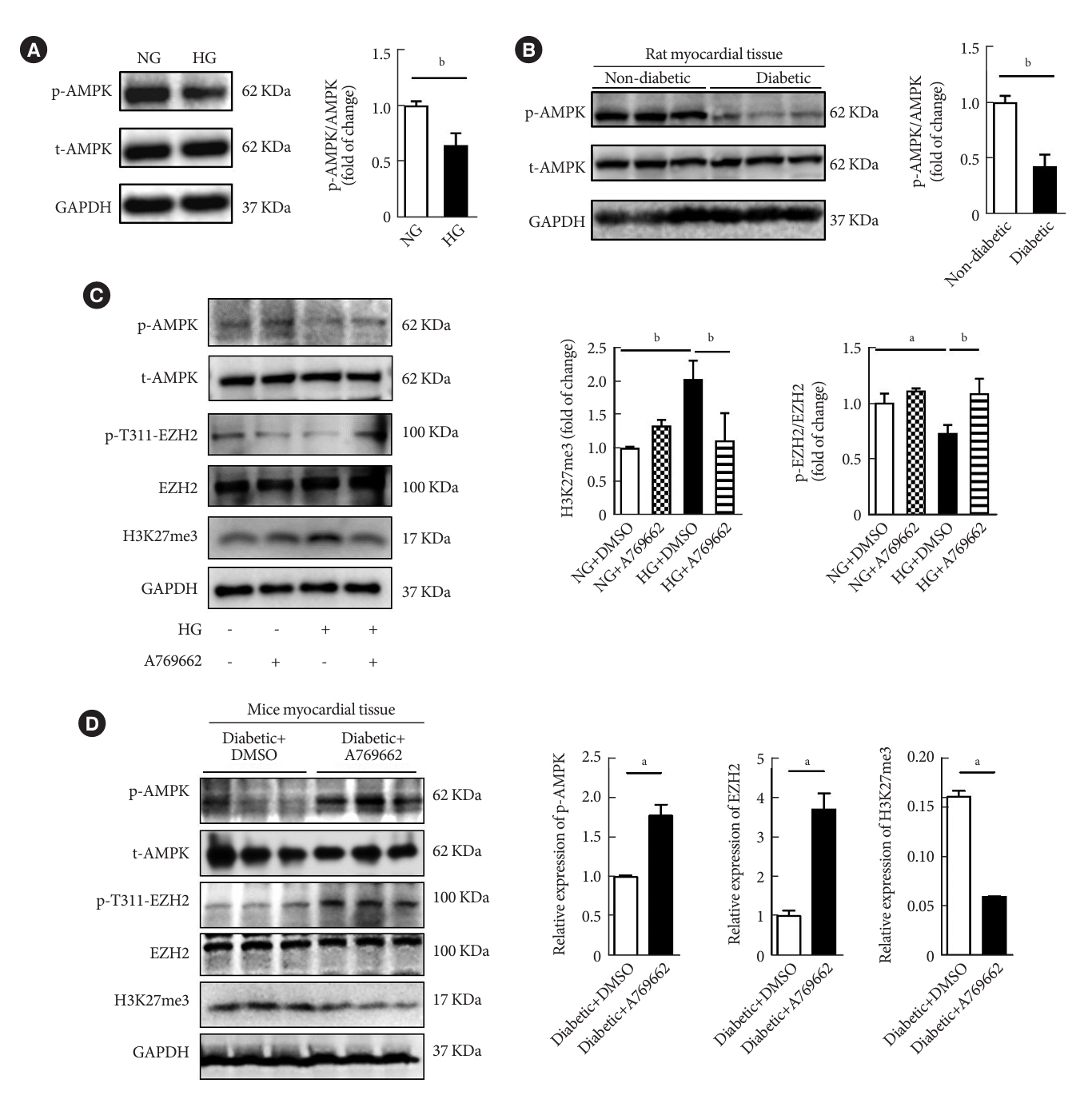
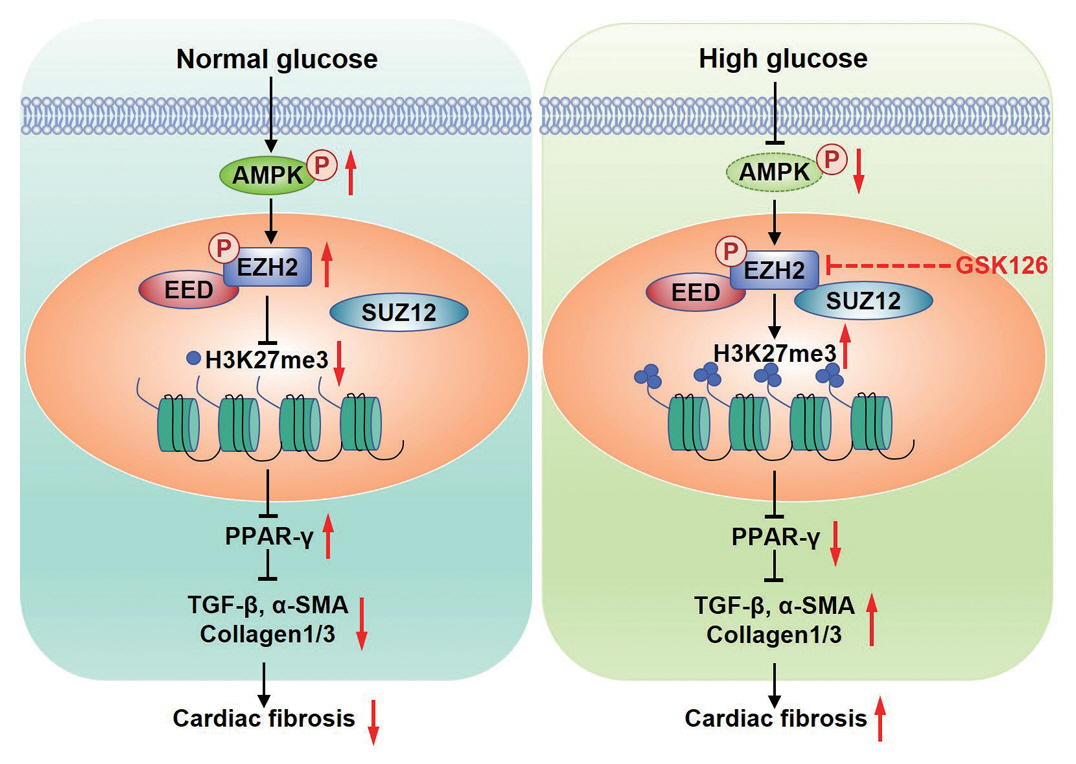
In STZ-induced diabetic ventricular tissues and HG-induced primary ventricular fibroblasts in vitro, H3K27 trimethylation was increased and the phosphorylation of EZH2 was reduced. Inhibition of EZH2 with GSK126 suppressed the activation, differentiation, and migration of cardiac fibroblasts as well as the overexpression of the fibrotic proteins induced by HG. Mechanical study demonstrated that HG reduced phosphorylation of EZH2 on Thr311 by inactivating AMP-activated protein kinase (AMPK), which transcriptionally inhibited peroxisome proliferator-activated receptor γ (PPAR-γ) expression to promote the fibroblasts activation and differentiation.
Conclusion
Our data revealed an AMPK/EZH2/PPAR-γ signal pathway is involved in HG-induced cardiac fibrosis.
Keyword
Figure
Cited by 2 articles
-
Targeting Cardiac Fibrosis in Diabetic Heart Failure: The Role of the EZH2, AMPK, and PPAR-γ Pathways (
Diabetes Metab J 2024;48:716-29)
Jooyeop Lee, Joon Ho Moon
Diabetes Metab J. 2024;48(6):1176-1178. doi: 10.4093/dmj.2024.0551.Diabetes Promotes Myocardial Fibrosis via AMPK/EZH2/PPAR-γ Signaling Pathway (<i>Diabetes Metab J</i> 2024;48:716-29)
Shan-Shan Li, Lu Pan, Shipeng Dang, Ru-Xing Wang
Diabetes Metab J. 2024;48(6):1181-1182. doi: 10.4093/dmj.2024.0675.
Reference
-
1. Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018; 61:21–8.
Article2. Jiang L, Wang J, Liu X, Li ZL, Xia CC, Xie LJ, et al. The combined effects of cardiac geometry, microcirculation, and tissue characteristics on cardiac systolic and diastolic function in subclinical diabetes mellitus-related cardiomyopathy. Int J Cardiol. 2020; 320:112–8.3. Che H, Wang Y, Li H, Li Y, Sahil A, Lv J, et al. Melatonin alleviates cardiac fibrosis via inhibiting lncRNA MALAT1/miR-141-mediated NLRP3 inflammasome and TGF-β1/Smads signaling in diabetic cardiomyopathy. FASEB J. 2020; 34:5282–98.
Article4. Tarbit E, Singh I, Peart JN, Rose’Meyer RB. Biomarkers for the identification of cardiac fibroblast and myofibroblast cells. Heart Fail Rev. 2019; 24:1–15.
Article5. Valiente-Alandi I, Potter SJ, Salvador AM, Schafer AE, Schips T, Carrillo-Salinas F, et al. Inhibiting fibronectin attenuates fibrosis and improves cardiac function in a model of heart failure. Circulation. 2018; 138:1236–52.
Article6. Khalil H, Kanisicak O, Prasad V, Correll RN, Fu X, Schips T, et al. Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest. 2017; 127:3770–83.
Article7. Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: the fibroblast awakens. Circ Res. 2016; 118:1021–40.8. Yang F, Qin Y, Lv J, Wang Y, Che H, Chen X, et al. Silencing long non-coding RNA Kcnq1ot1 alleviates pyroptosis and fibrosis in diabetic cardiomyopathy. Cell Death Dis. 2018; 9:1000.
Article9. Felisbino MB, McKinsey TA. Epigenetics in cardiac fibrosis: emphasis on inflammation and fibroblast activation. JACC Basic Transl Sci. 2018; 3:704–15.10. Laugesen A, Hojfeldt JW, Helin K. Molecular mechanisms directing PRC2 recruitment and H3K27 methylation. Mol Cell. 2019; 74:8–18.
Article11. Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. J Hematol Oncol. 2020; 13:104.
Article12. Wan L, Xu K, Wei Y, Zhang J, Han T, Fry C, et al. Phosphorylation of EZH2 by AMPK suppresses PRC2 methyltransferase activity and oncogenic function. Mol Cell. 2018; 69:279–91.13. Tsou PS, Campbell P, Amin MA, Coit P, Miller S, Fox DA, et al. Inhibition of EZH2 prevents fibrosis and restores normal angiogenesis in scleroderma. Proc Natl Acad Sci U S A. 2019; 116:3695–702.
Article14. Coward WR, Brand OJ, Pasini A, Jenkins G, Knox AJ, Pang L. Interplay between EZH2 and G9a regulates CXCL10 gene repression in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2018; 58:449–60.
Article15. Lau-Corona D, Bae WK, Hennighausen L, Waxman DJ. Sex-biased genetic programs in liver metabolism and liver fibrosis are controlled by EZH1 and EZH2. PLoS Genet. 2020; 16:e1008796.
Article16. Haye A, Ansari MA, Rahman SO, Shamsi Y, Ahmed D, Sharma M. Role of AMP-activated protein kinase on cardio-metabolic abnormalities in the development of diabetic cardiomyopathy: a molecular landscape. Eur J Pharmacol. 2020; 888:173376.
Article17. Sun Y, Zhou S, Guo H, Zhang J, Ma T, Zheng Y, et al. Protective effects of sulforaphane on type 2 diabetes-induced cardiomyopathy via AMPK-mediated activation of lipid metabolic pathways and NRF2 function. Metabolism. 2020; 102:154002.18. Qi H, Liu Y, Li S, Chen Y, Li L, Cao Y, et al. Activation of AMPK attenuated cardiac fibrosis by inhibiting CDK2 via p21/p27 and miR-29 family pathways in rats. Mol Ther Nucleic Acids. 2017; 8:277–90.
Article19. Ma ZG, Yuan YP, Zhang X, Xu SC, Wang SS, Tang QZ. Piperine attenuates pathological cardiac fibrosis via PPAR-γ/AKT pathways. EBioMedicine. 2017; 18:179–87.
Article20. Gong K, Chen YF, Li P, Lucas JA, Hage FG, Yang Q, et al. Transforming growth factor-β inhibits myocardial PPARγ expression in pressure overload-induced cardiac fibrosis and remodeling in mice. J Hypertens. 2011; 29:1810–9.
Article21. Hou X, Zhang Y, Shen YH, Liu T, Song S, Cui L, et al. PPAR-γ activation by rosiglitazone suppresses angiotensin II-mediated proliferation and phenotypictransition in cardiac fibroblasts via inhibition of activation of activator protein 1. Eur J Pharmacol. 2013; 715:196–203.
Article22. Qi HP, Wang Y, Zhang QH, Guo J, Li L, Cao YG, et al. Activation of peroxisome proliferator-activated receptor γ (PPARγ) through NF-κB/Brg1 and TGF-β1 pathways attenuates cardiac remodeling in pressure-overloaded rat hearts. Cell Physiol Biochem. 2015; 35:899–912.23. Teunissen BE, Smeets PJ, Willemsen PH, De Windt LJ, Van der Vusse GJ, Van Bilsen M. Activation of PPARdelta inhibits cardiac fibroblast proliferation and the transdifferentiation into myofibroblasts. Cardiovasc Res. 2007; 75:519–29.24. Zhang ZY, Qian LL, Wang N, Miao LF, Ma X, Dang SP, et al. Glucose fluctuations promote vascular BK channels dysfunction via PKCα/NF-κB/MuRF1 signaling. J Mol Cell Cardiol. 2020; 145:14–24.
Article25. Gao W, Guo N, Zhao S, Chen Z, Zhang W, Yan F, et al. FBXW7 promotes pathological cardiac hypertrophy by targeting EZH2-SIX1 signaling. Exp Cell Res. 2020; 393:112059.
Article26. Pursani V, Pethe P, Bashir M, Sampath P, Tanavde V, Bhartiya D. Genetic and epigenetic profiling reveals EZH2-mediated down regulation of OCT-4 involves NR2F2 during cardiac differentiation of human embryonic stem cells. Sci Rep. 2017; 7:13051.
Article27. Hu H, Wu J, Yu X, Zhou J, Yu H, Ma L. Long non-coding RNA MALAT1 enhances the apoptosis of cardiomyocytes through autophagy inhibition by regulating TSC2-mTOR signaling. Biol Res. 2019; 52:58.28. Yue Z, Chen J, Lian H, Pei J, Li Y, Chen X, et al. PDGFR-β signaling regulates cardiomyocyte proliferation and myocardial regeneration. Cell Rep. 2019; 28:966–78.
Article29. Ai S, Yu X, Li Y, Peng Y, Li C, Yue Y, et al. Divergent requirements for EZH1 in heart development versus regeneration. Circ Res. 2017; 121:106–12.
Article30. Song S, Zhang R, Mo B, Chen L, Liu L, Yu Y, et al. EZH2 as a novel therapeutic target for atrial fibrosis and atrial fibrillation. J Mol Cell Cardiol. 2019; 135:119–33.
Article31. Zhao L, You T, Lu Y, Lin S, Li F, Xu H. Elevated EZH2 in ischemic heart disease epigenetically mediates suppression of NaV1.5 expression. J Mol Cell Cardiol. 2021; 153:95–103.
Article32. Meng XD, Yao HH, Wang LM, Yu M, Shi S, Yuan ZX, et al. Knockdown of GAS5 inhibits atherosclerosis progression via reducing EZH2-mediated ABCA1 transcription in ApoE-/- mice. Mol Ther Nucleic Acids. 2020; 19:84–96.33. Wang C, Liu G, Yang H, Guo S, Wang H, Dong Z, et al. MALAT1-mediated recruitment of the histone methyltransferase EZH2 to the microRNA-22 promoter leads to cardiomyocyte apoptosis in diabetic cardiomyopathy. Sci Total Environ. 2021; 766:142191.
Article34. Xiao X, Senavirathna LK, Gou X, Huang C, Liang Y, Liu L. EZH2 enhances the differentiation of fibroblasts into myofibroblasts in idiopathic pulmonary fibrosis. Physiol Rep. 2016; 4:e12915.
Article35. Zhu WS, Tang CM, Xiao Z, Zhu JN, Lin QX, Fu YH, et al. Targeting EZH1 and EZH2 contributes to the suppression of fibrosis-associated genes by miR-214-3p in cardiac myofibroblasts. Oncotarget. 2016; 7:78331–42.
Article36. Shih YC, Chen CL, Zhang Y, Mellor RL, Kanter EM, Fang Y, et al. Endoplasmic reticulum protein TXNDC5 augments myocardial fibrosis by facilitating extracellular matrix protein folding and redox-sensitive cardiac fibroblast activation. Circ Res. 2018; 122:1052–68.
Article37. Nagaraju CK, Robinson EL, Abdesselem M, Trenson S, Dries E, Gilbert G, et al. Myofibroblast phenotype and reversibility of fibrosis in patients with end-stage heart failure. J Am Coll Cardiol. 2019; 73:2267–82.38. Cho N, Razipour SE, McCain ML. Featured article: TGF-β1 dominates extracellular matrix rigidity for inducing differentiation of human cardiac fibroblasts to myofibroblasts. Exp Biol Med (Maywood). 2018; 243:601–12.
Article39. Li M, Hong W, Hao C, Li L, Wu D, Shen A, et al. SIRT1 antagonizes liver fibrosis by blocking hepatic stellate cell activation in mice. FASEB J. 2018; 32:500–11.
Article40. Kalliora C, Kyriazis ID, Oka SI, Lieu MJ, Yue Y, Area-Gomez E, et al. Dual peroxisome-proliferator-activated-receptor-α/γ activation inhibits SIRT1-PGC1α axis and causes cardiac dysfunction. JCI Insight. 2019; 5:e129556.41. Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018; 122:624–38.42. Mann J, Chu DC, Maxwell A, Oakley F, Zhu NL, Tsukamoto H, et al. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology. 2010; 138:705–14.43. Qi D, Young LH. AMPK: energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol Metab. 2015; 26:422–9.
Article44. Lin SC, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Cell Metab. 2018; 27:299–313.
Article45. Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPKmediated inhibition of mitochondrial fission. Redox Biol. 2018; 15:335–46.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diabetes Promotes Myocardial Fibrosis via AMPK/EZH2/PPAR-γ Signaling Pathway (Diabetes Metab J 2024;48:716-29)
- Targeting Cardiac Fibrosis in Diabetic Heart Failure: The Role of the EZH2, AMPK, and PPAR-γ Pathways (Diabetes Metab J 2024;48:716-29)
- AMPK and Exercise: Glucose Uptake and Insulin Sensitivity
- The Effect of AMPK Activation on Wnt and sFRP5 in TNF-alpha Induced Adipocyte Metabolic Dysfunction in 3T3-L1 Cell
- Peiminine inhibits myocardial injury and fibrosis after myocardial infarction in rats by regulating mitogen-activated protein kinase pathway