J Pathol Transl Med.
2024 Jul;58(4):174-181. 10.4132/jptm.2024.05.02.
Immunohistochemical expression in idiopathic inflammatory myopathies at a single center in Vietnam
- Affiliations
-
- 1Department of Pathology, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
- 2Neurology Center, University Medical Center Ho Chi Minh City, Ho Chi Minh City, Vietnam
- 3Neurology Center, International NeuroSurgery Hospital, Ho Chi Minh City, Vietnam
- KMID: 2557757
- DOI: http://doi.org/10.4132/jptm.2024.05.02
Abstract
- Background
The identification of idiopathic inflammatory myopathies (IIMs) requires a comprehensive analysis involving clinical manifestations and histological findings. This study aims to provide insights into the histopathological and immunohistochemical aspects of IIMs.
Methods
This retrospective case series involved 56 patients diagnosed with IIMs at the Department of Pathology, University of Medicine and Pharmacy at Ho Chi Minh City, from 2019 to 2023. The histology and immunohistochemical expression of HLA-ABC, HLA-DR, C5b-9, Mx1/2/3, and p62 were detected.
Results
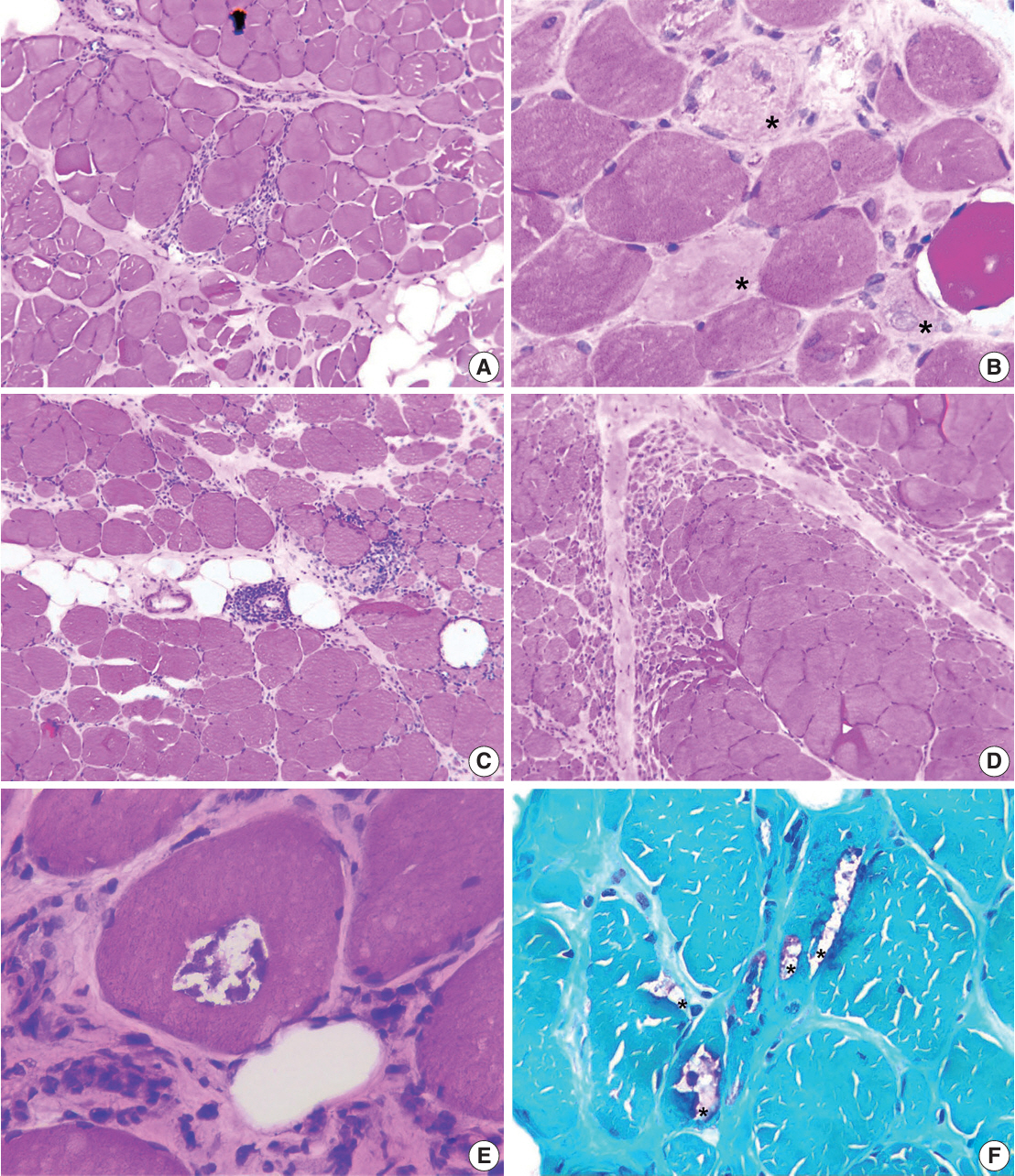
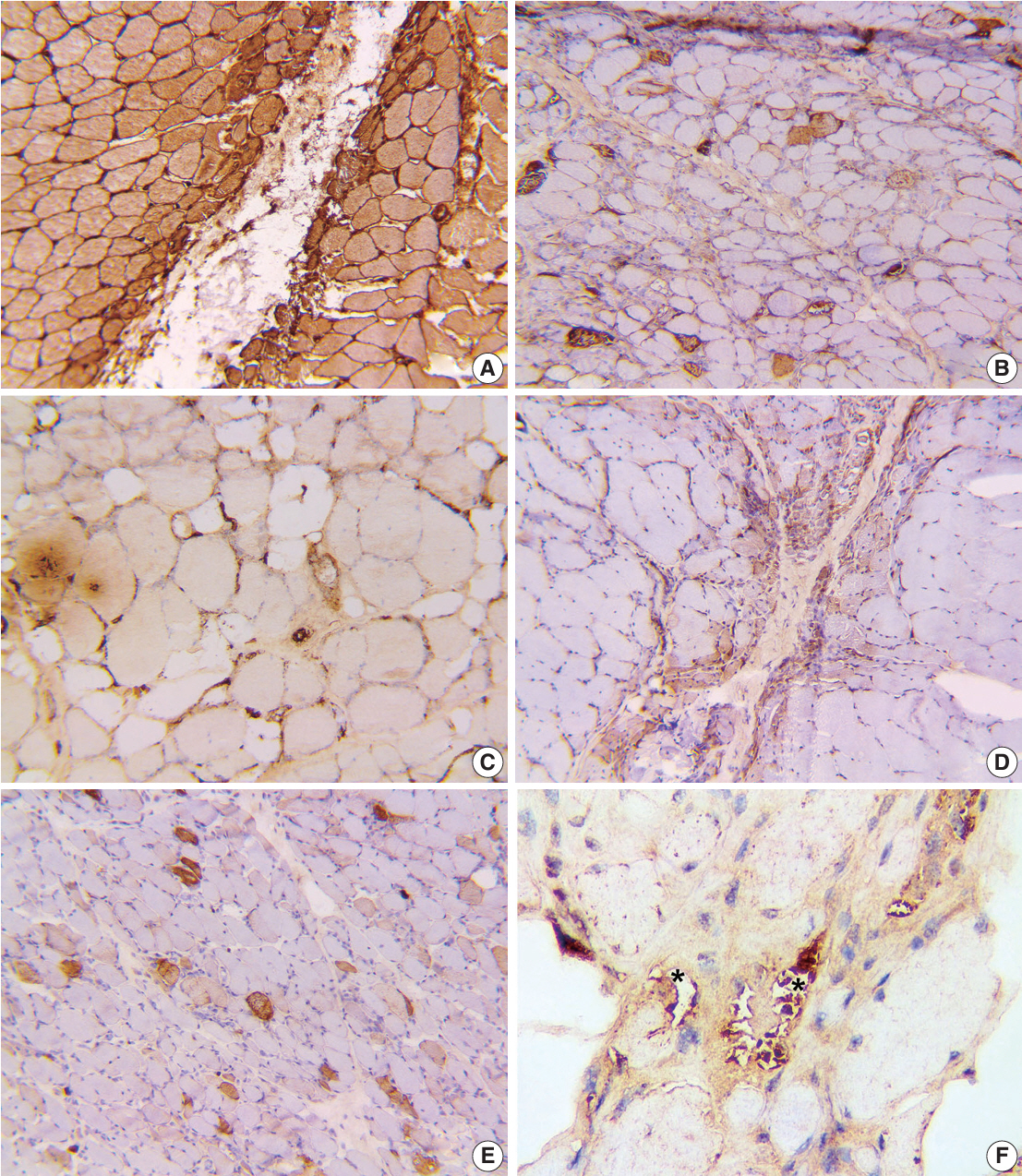
We examined six categories of inflammatory myopathy, including immunemediated necrotizing myopathy (58.9%), dermatomyositis (DM; 23.2%), overlap myositis (8.9%), antisynthetase syndrome (5.4%), inclusion body myositis (IBM; 1.8%), and polymyositis (1.8%). The average age of the patients was 49.7 ± 16.1 years, with a female-to-male ratio of 3:1. Inflammatory cell infiltration in the endomysium was present in 62.5% of cases, perifascicular atrophy was found in 17.8%, and fiber necrosis was observed in 42 cases (75.0%). Rimmed vacuoles were present in 100% of cases in the IBM group. Immunohistochemistry showed the following positivity rates: HLA-ABC (89.2%), HLA-DR (19.6%), C5b-9 (57.1%), and Mx1/2/3 (10.7%). Mx1/2/3 expression was high in DM cases. p62 vacuole deposits were noted in the IBM case. The combination of membrane attack complex and major histocompatibility complex I helped detect IIMs in 96% of cases.
Conclusions
The diagnosis of IIMs and their subtypes should be based on clinical features and histopathological characteristics. Immunohistochemistry plays a crucial role in the diagnosis and differentiation of these subgroups.
Keyword
Figure
Reference
-
References
1. Mammen AL, Allenbach Y, Stenzel W, Benveniste O; ENMC 239th Workshop Study Group. 239th ENMC International Workshop: classification of dermatomyositis, Amsterdam, the Netherlands, 14-16 December 2018. Neuromuscul Disord. 2020; 30:70–92.
Article2. Allenbach Y, Mammen AL, Benveniste O, Stenzel W; ImmuneMediated Necrotizing Myopathies Working Group. 224th ENMC International Workshop: clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14-16 October 2016. Neuromuscul Disord. 2018; 28:87–99.3. Rose MR; ENMC IBM Working Group. 188th ENMC International Workshop: inclusion body myositis, 2-4 December 2011, Naarden, The Netherlands. Neuromuscul Disord. 2013; 23:1044–55.
Article4. Watanabe Y, Uruha A, Suzuki S, et al. Clinical features and prognosis in anti-SRP and anti-HMGCR necrotising myopathy. J Neurol Neurosurg Psychiatry. 2016; 87:1038–44.
Article5. Gupta L, Naveen R, Gaur P, Agarwal V, Aggarwal R. Myositis-specific and myositis-associated autoantibodies in a large Indian cohort of inflammatory myositis. Semin Arthritis Rheum. 2021; 51:113–20.
Article6. Tun ZP, Nyunt CC, et al. Profile of various idiopathic inflammatory myopathies at two university hospitals in Yangon, Myanmar. Neurol Asia. 2020; 25:285–91.7. Chen Z, Hu W, Wang Y, Guo Z, Sun L, Kuwana M. Distinct profiles of myositis-specific autoantibodies in Chinese and Japanese patients with polymyositis/dermatomyositis. Clin Rheumatol. 2015; 34:1627–31.8. van der Meulen MF, Bronner IM, Hoogendijk JE, et al. Polymyositis: an overdiagnosed entity. Neurology. 2003; 61:316–21.9. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975; 292:403–7.10. Uruha A, Suzuki S, Nishino I. Diagnosis of dermatomyositis: autoantibody profile and muscle pathology. Neuroimmunology. 2017; 8:302–12.
Article11. Inoue M, Tanboon J, Okubo M, et al. Absence of sarcoplasmic myxovirus resistance protein A (MxA) expression in antisynthetase syndrome in a cohort of 194 cases. Neuropathol Appl Neurobiol. 2019; 45:523–4.
Article12. Noguchi E, Uruha A, Suzuki S, et al. Skeletal muscle involvement in antisynthetase syndrome. JAMA Neurol. 2017; 74:992–9.13. Das L, Blumbergs PC, Manavis J, Limaye VS. Major histocompatibility complex class I and II expression in idiopathic inflammatory myopathy. Appl Immunohistochem Mol Morphol. 2013; 21:539–42.
Article14. Rider LG, Koziol D, Giannini EH, et al. Validation of manual muscle testing and a subset of eight muscles for adult and juvenile idiopathic inflammatory myopathies. Arthritis Care Res (Hoboken). 2010; 62:465–72.
Article15. Uruha A, Goebel HH, Stenzel W. Updates on the Immunopathology in idiopathic inflammatory myopathies. Curr Rheumatol Rep. 2021; 23:56.
Article16. van der Pas J, Hengstman GJ, ter Laak HJ, Borm GF, van Engelen BG. Diagnostic value of MHC class I staining in idiopathic inflammatory myopathies. J Neurol Neurosurg Psychiatry. 2004; 75:136–9.17. Confalonieri P, Oliva L, Andreetta F, et al. Muscle inflammation and MHC class I up-regulation in muscular dystrophy with lack of dysferlin: an immunopathological study. J Neuroimmunol. 2003; 142:130–6.18. Rodriguez Cruz PM, Luo YB, Miller J, Junckerstorff RC, Mastaglia FL, Fabian V. An analysis of the sensitivity and specificity of MHC-I and MHC-II immunohistochemical staining in muscle biopsies for the diagnosis of inflammatory myopathies. Neuromuscul Disord. 2014; 24:1025–35.
Article19. Choi JH, Park YE, Kim SI, et al. Differential immunohistological features of inflammatory myopathies and dysferlinopathy. J Korean Med Sci. 2009; 24:1015–23.
Article20. Sakuta R, Murakami N, Jin Y, Nagai T, Nonaka I, Nishino I. Diagnostic significance of membrane attack complex and vitronectin in childhood dermatomyositis. J Child Neurol. 2005; 20:597–602.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increased Interleukin-17 Expression in Patients with Idiopathic Inflammatory Myopathies
- The Expression of Toll-like Receptors in Idiopathic Inflammatory Myopathies
- Extensive inflammatory reaction in facioscapulohumeral muscular dystrophy
- The Laboratory Test for the Diagnosis of Idiopathic Inflammatory Myopathies
- Differential Immunohistological Features of Inflammatory Myopathies and Dysferlinopathy