Korean J Pain.
2024 Jul;37(3):211-217. 10.3344/kjp.24066.
Beneficial effect of metformin on tolerance to analgesic effects of sodium salicylate in male rats
- Affiliations
-
- 1Department of Physiology, School of Medicine, Torbat Heydariyeh University of Medical Sciences, Torbat Heydariyeh, Iran
- 2Department of Biology and Microbiology, School of Medical Laboratory Technology, Khatam Al-Nabieen University, Kabul, Afghanistan
- 3Department of Physiology, School of Medicine, Arak University of Medical Sciences, Arak, Iran
- KMID: 2557725
- DOI: http://doi.org/10.3344/kjp.24066
Abstract
- Background
Tolerance to the analgesic effects of opioids and non-steroidal anti-inflammatory drugs (NSAIDs) is a major concern for relieving pain. Thus, it is highly valuable to find new pharmacological strategies for prolonged therapeutic procedures. Biguanide-type drugs such as metformin (MET) are effective for neuroprotection and can be beneficial for addressing opioid tolerance in the treatment of chronic pain. It has been proposed that analgesic tolerance to NSAIDs is mediated by the endogenous opioid system. According to the cross-tolerance between NSAIDs, especially sodium salicylate (SS), and opiates, especially morphine, the objective of this study was to investigate whether MET administration can reduce tolerance to the anti-nociceptive effects of SS.
Methods
Fifty-six male Wistar rats were used in this research (weight 200–250 g). For induction of tolerance, SS (300 mg/kg) was injected intraperitoneally for 7 days. During the examination period, animals received MET at doses of 50, 75, or 100 mg/kg for 7 days to evaluate the development of tolerance to the analgesic effect of SS. The hot plate test was used to evaluate the drugs' anti-nociceptive properties.
Results
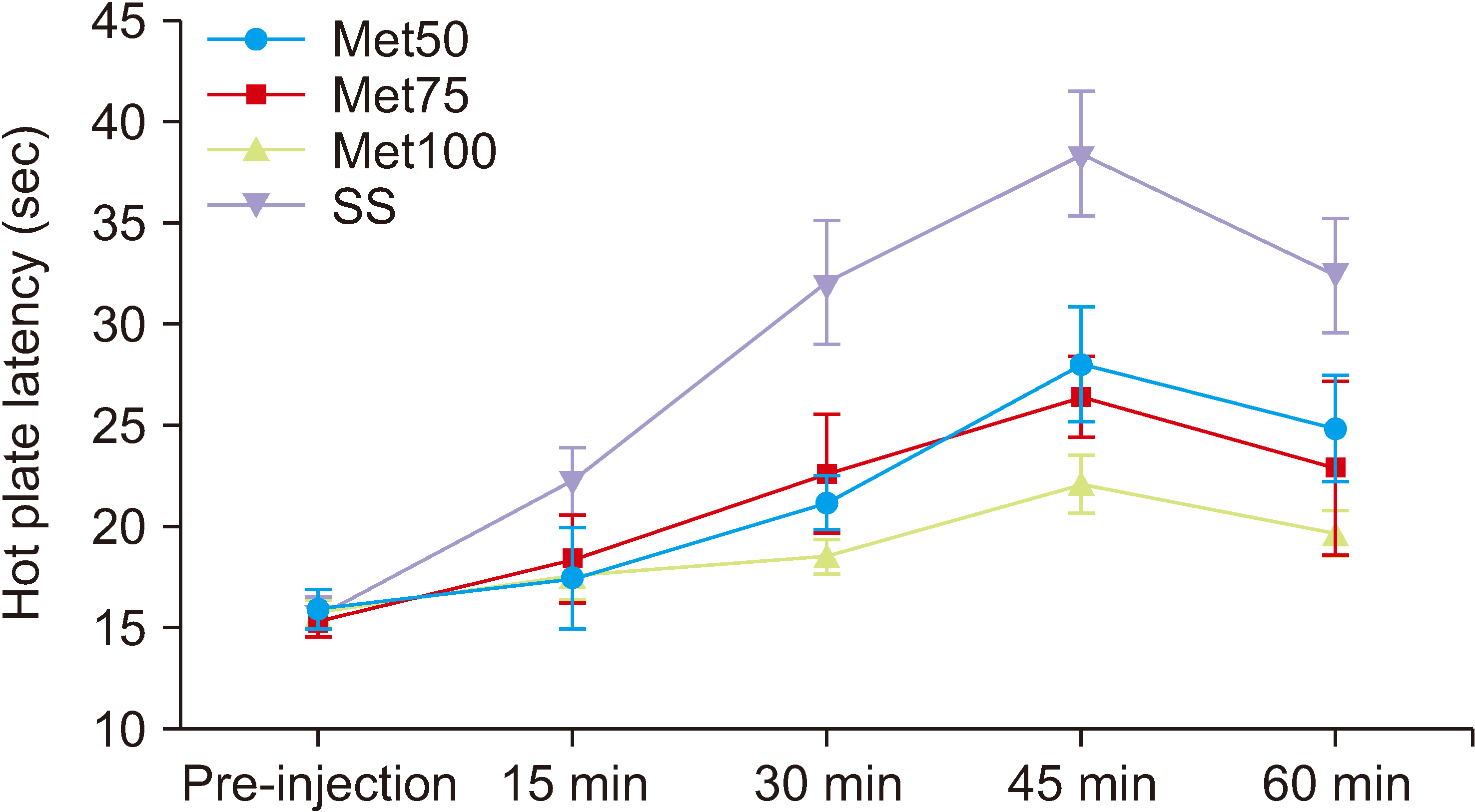
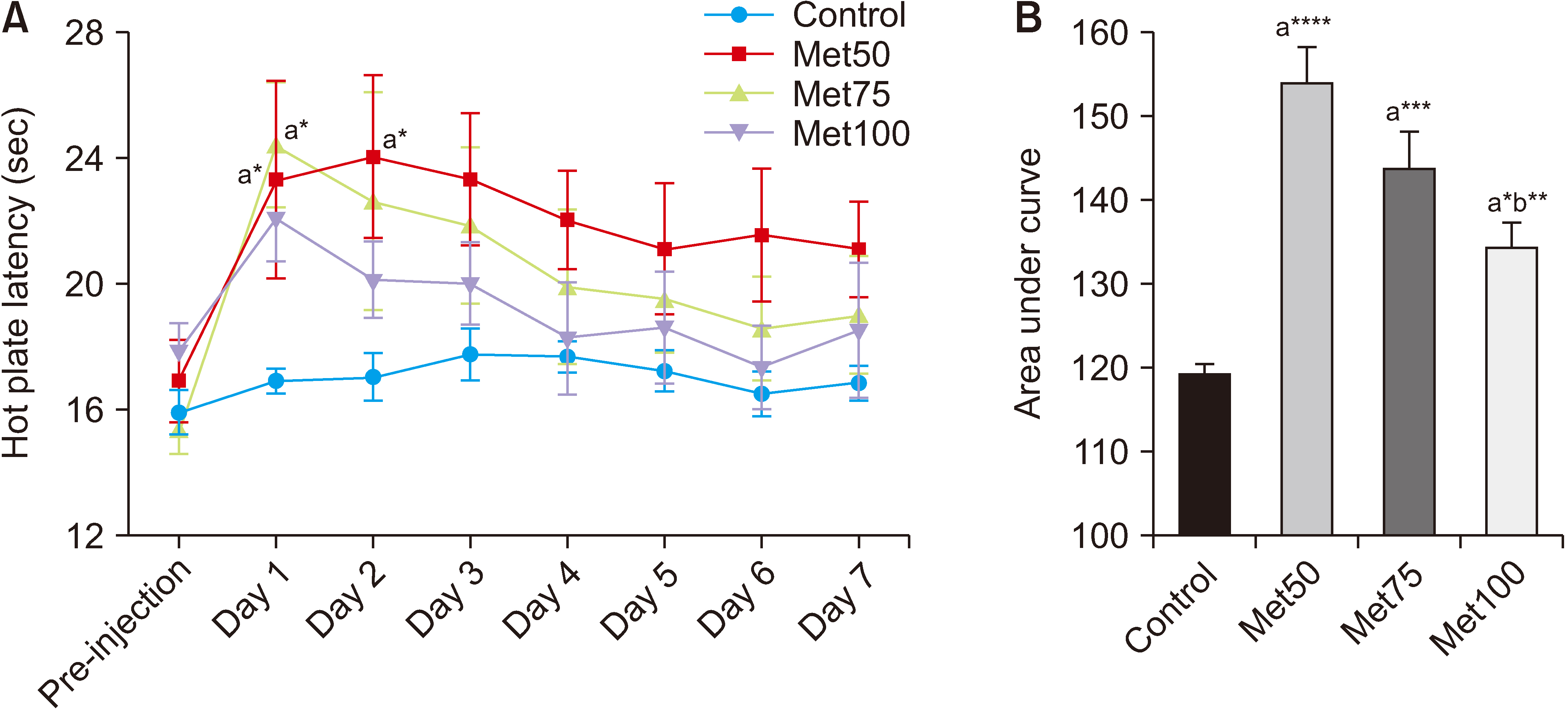
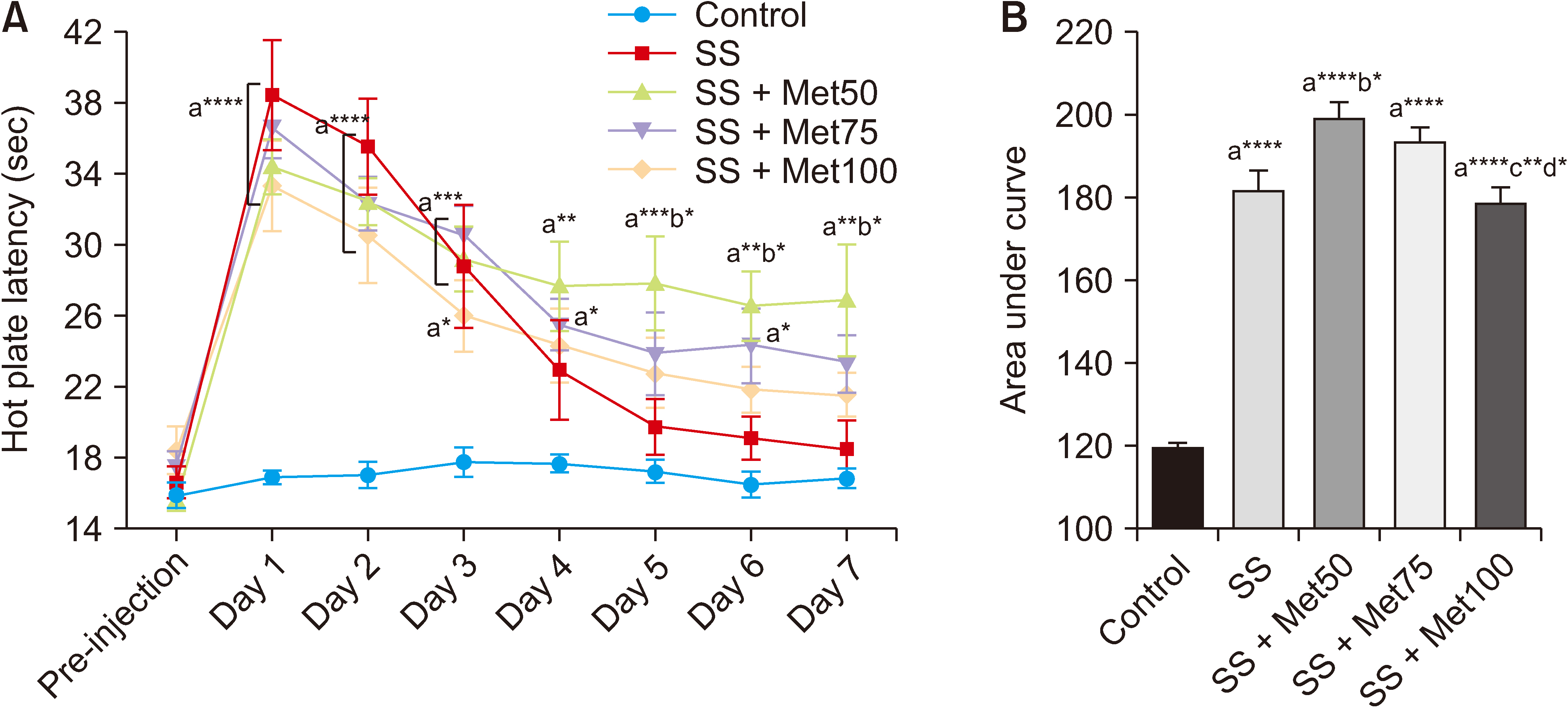
Salicylate injection significantly increased hot plate latency as compared to the control group, but the total analgesic effect of co-treatment with SS + Met50 was stronger than the SS group. Furthermore, the effect of this combination undergoes less analgesic tolerance over time.
Conclusions
It can be concluded that MET can reduce the analgesic tolerance that is induced by repeated intraperitoneal injections of SS in Wister rats.
Keyword
Figure
Reference
-
1. Pan Y, Sun X, Jiang L, Hu L, Kong H, Han Y, et al. 2016; Metformin reduces morphine tolerance by inhibiting microglial-mediated neuroinflammation. J Neuroinflammation. 13:294. DOI: 10.1186/s12974-016-0754-9. PMID: 27855689. PMCID: PMC5114746.2. Déciga-Campos M, López UG, Reval MI, López-Muñoz FJ. 2003; Enhancement of antinociception by co-administration of an opioid drug (morphine) and a preferential cyclooxygenase-2 inhibitor (rofecoxib) in rats. Eur J Pharmacol. 460:99–107. DOI: 10.1016/S0014-2999(02)02920-5. PMID: 12559369.
Article3. Matsuda M, Huh Y, Ji RR. 2019; Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth. 33:131–9. DOI: 10.1007/s00540-018-2579-4. PMID: 30448975. PMCID: PMC6813778.4. Owoyele BV, Bakare AO, Olaseinde OF, Ochu MJ, Yusuff AM, Ekebafe F, et al. 2022; Corrigendum: synergistic interaction between acetaminophen and L-carnosine improved neuropathic pain via NF-κB pathway and antioxidant properties in chronic constriction injury model. Korean J Pain. 35:488. Erratum for: Korean J Pain 2022; 35: 271. DOI: 10.3344/kjp.2022.35.4.488. PMID: 36175348. PMCID: PMC9530685.
Article5. Pernia-Andrade AJ, Tortorici V, Vanegas H. 2004; Induction of opioid tolerance by lysine-acetylsalicylate in rats. Pain. 111:191–200. DOI: 10.1016/j.pain.2004.06.006. PMID: 15327823.
Article6. Wideman GL, Keffer M, Morris E, Doyle RT Jr, Jiang JG, Beaver WT. 1999; Analgesic efficacy of a combination of hydrocodone with ibuprofen in postoperative pain. Clin Pharmacol Ther. 65:66–76. DOI: 10.1016/S0009-9236(99)70123-2. PMID: 9951432.
Article7. Sunshine A, Olson NZ, O'Neill E, Ramos I, Doyle R. 1997; Analgesic efficacy of a hydrocodone with ibuprofen combination compared with ibuprofen alone for the treatment of acute postoperative pain. J Clin Pharmacol. 37:908–15. DOI: 10.1002/j.1552-4604.1997.tb04265.x. PMID: 9505982.
Article8. Christie MJ, Vaughan CW, Ingram SL. 1999; Opioids, NSAIDs and 5-lipoxygenase inhibitors act synergistically in brain via arachidonic acid metabolism. Inflamm Res. 48:1–4. DOI: 10.1007/s000110050367. PMID: 9987677.
Article9. Tsiklauri N, Gurtskaia G, Tsagareli M. 2008; Study of non-opioid analgesics tolerance in young and adult rats. Georgian Med News. 158:40–4.10. Trujillo KA, Akil H. 1991; Opiate tolerance and dependence: recent findings and synthesis. New Biol. 3:915–23.11. Bao W, Luo Y, Wang D, Li J, Wu X, Mei W. 2018; Sodium salicylate modulates inflammatory responses through AMP-activated protein kinase activation in LPS-stimulated THP-1 cells. J Cell Biochem. 119:850–60. DOI: 10.1002/jcb.26249. PMID: 28661045. PMCID: PMC5724678.
Article12. LaMoia TE, Shulman GI. 2021; Cellular and molecular mechanisms of metformin action. Endocr Rev. 42:77–96. DOI: 10.1210/endrev/bnaa023. PMID: 32897388. PMCID: PMC7846086.
Article13. Liu SN, Liu Q, Sun SJ, Hou SC, Wang Y, Shen ZF. 2014; [Metformin ameliorates β-cell dysfunction by regulating inflammation production, ion and hormone homeostasis of pancreas in diabetic KKAy mice]. Yao Xue Xue Bao. 49:1554–62. Chinese.14. Zhou C, Sun R, Zhuang S, Sun C, Jiang Y, Cui Y, et al. 2016; Metformin prevents cerebellar granule neurons against glutamate-induced neurotoxicity. Brain Res Bull. 121:241–5. DOI: 10.1016/j.brainresbull.2016.02.009. PMID: 26876755.
Article15. Bonnefont-Rousselot D, Raji B, Walrand S, Gardès-Albert M, Jore D, Legrand A, Peynet J, et al. 2003; An intracellular modulation of free radical production could contribute to the beneficial effects of metformin towards oxidative stress. Metabolism. 52:586–9. DOI: 10.1053/meta.2003.50093. PMID: 12759888.
Article16. Bułdak Ł, Machnik G, Bułdak RJ, Łabuzek K, Bołdys A, Okopień B. 2016; Exenatide and metformin express their anti-inflammatory effects on human monocytes/macrophages by the attenuation of MAPKs and NFκB signaling. Naunyn Schmiedebergs Arch Pharmacol. 389:1103–15. DOI: 10.1007/s00210-016-1277-8. PMID: 27424158.
Article17. Houser VP, Paré WP. 1973; Analgesic potency of sodium salicylate, indomethacin, and chlordiazepoxide as measured by the spatial preference technique in the rat. Psychopharmacologia. 32:121–31. DOI: 10.1007/BF00428683. PMID: 4753530.
Article18. Sadegh M, Fathollahi Y, Naghdi N, Semnanian S. 2013; Morphine deteriorates spatial memory in sodium salicylate treated rats. Eur J Pharmacol. 704:1–6. DOI: 10.1016/j.ejphar.2013.02.017. PMID: 23461854.
Article19. Afshari K, Dehdashtian A, Haddadi NS, Haj-Mirzaian A, Iranmehr A, Ebrahimi MA, et al. 2018; Anti-inflammatory effects of Metformin improve the neuropathic pain and locomotor activity in spinal cord injured rats: introduction of an alternative therapy. Spinal Cord. 56:1032–41. DOI: 10.1038/s41393-018-0168-x. PMID: 29959433.
Article20. Pecikoza UB, Tomić MA, Micov AM, Stepanović-Petrović RM. 2017; Metformin synergizes with conventional and adjuvant analgesic drugs to reduce inflammatory hyperalgesia in rats. Anesth Analg. 124:1317–29. DOI: 10.1213/ANE.0000000000001561. PMID: 27669556.
Article21. Tsiklauri N, Pirkulashvili N, Nozadze I, Nebieridze M, Gurtskaia G, Abzianidze E, et al. 2018; Antinociceptive tolerance to NSAIDs in the anterior cingulate cortex is mediated via endogenous opioid mechanism. BMC Pharmacol Toxicol. 19:2. DOI: 10.1186/s40360-017-0193-y. PMID: 29304875. PMCID: PMC5756434.
Article22. Giglio CA, Defino HL, Del Bel EA. da-Silva CA. de-Souza AS. 2006; Behavioral and physiological methods for early quantitative assessment of spinal cord injury and prognosis in rats. Braz J Med Biol Res. 39:1613–23. DOI: 10.1590/S0100-879X2006001200013. PMID: 17160271.
Article23. Rena G, Hardie DG, Pearson ER. 2017; The mechanisms of action of metformin. Diabetologia. 60:1577–85. DOI: 10.1007/s00125-017-4342-z. PMID: 28776086. PMCID: PMC5552828.
Article24. Asiedu MN, Han C, Dib-Hajj SD, Waxman SG, Price TJ, Dussor G. 2017; The AMPK activator A769662 blocks voltage-gated sodium channels: discovery of a novel pharmacophore with potential utility for analgesic development. PLoS One. 12:e0169882. DOI: 10.1371/journal.pone.0169882. PMID: 28118359. PMCID: PMC5261566.
Article25. Baeza-Flores GDC, Guzmán-Priego CG, Parra-Flores LI, Murbartián J, Torres-López JE, Granados-Soto V. 2020; Metformin: a prospective alternative for the treatment of chronic pain. Front Pharmacol. 11:558474. DOI: 10.3389/fphar.2020.558474. PMID: 33178015. PMCID: PMC7538784.
Article26. Russe OQ, Möser CV, Kynast KL, King TS, Stephan H, Geisslinger G, et al. 2013; Activation of the AMP-activated protein kinase reduces inflammatory nociception. J Pain. 14:1330–40. DOI: 10.1016/j.jpain.2013.05.012. PMID: 23916727.
Article27. Bullón P, Alcocer-Gómez E, Carrión AM, Marín-Aguilar F, Garrido-Maraver J, Román-Malo L, et al. 2016; AMPK phosphorylation modulates pain by activation of NLRP3 inflammasome. Antioxid Redox Signal. 24:157–70. DOI: 10.1089/ars.2014.6120. PMID: 26132721. PMCID: PMC4742979.
Article28. Augusto PSA, Braga AV, Rodrigues FF, Morais MI, Dutra MMGB, Batista CRA, et al. 2019; Metformin antinociceptive effect in models of nociceptive and neuropathic pain is partially mediated by activation of opioidergic mechanisms. Eur J Pharmacol. 858:172497. DOI: 10.1016/j.ejphar.2019.172497. PMID: 31238066.
Article29. Riddle M. 2000; Combining sulfonylureas and other oral agents. Am J Med. 108(Suppl 6a):15S–22S. DOI: 10.1016/S0002-9343(00)00338-7. PMID: 10764846.30. Burian M, Geisslinger G. 2005; COX-dependent mechanisms involved in the antinociceptive action of NSAIDs at central and peripheral sites. Pharmacol Ther. 107:139–54. DOI: 10.1016/j.pharmthera.2005.02.004. PMID: 15993252.
Article31. Vanegas H, Schaible HG. 2001; Prostaglandins and cyclooxygenases [correction of cycloxygenases] in the spinal cord. Prog Neurobiol. 64:327–63. Erratum in: Prog Neurobiol 2001; 65: 609. DOI: 10.1016/S0301-0082(01)00026-0.32. Vanegas H, Tortorici V. 2002; Opioidergic effects of nonopioid analgesics on the central nervous system. Cell Mol Neurobiol. 22:655–61. DOI: 10.1023/A:1021896622089. PMID: 12585685.33. Elberry AA, Sharkawi SMZ, Wahba MR. 2019; Antinociceptive and anti-inflammatory effects of N-acetylcysteine and verapamil in Wistar rats. Korean J Pain. 32:256–63. DOI: 10.3344/kjp.2019.32.4.256. PMID: 31569917. PMCID: PMC6813896.
Article34. Tsiklauri N, Viatchenko-Karpinski V, Voitenko N, Tsagareli MG. 2010; Non-opioid tolerance in juvenile and adult rats. Eur J Pharmacol. 629:68–72. DOI: 10.1016/j.ejphar.2009.12.016. PMID: 20035744.
Article35. Shirooie S, Esmaeili J, Sureda A, Esmaeili N, Mirzaee Saffari P, Yousefi-Manesh H, et al. 2020; Evaluation of the effects of metformin administration on morphine tolerance in mice. Neurosci Lett. 716:134638. DOI: 10.1016/j.neulet.2019.134638. PMID: 31756370.36. Ji RR, Gereau RW 4th, Malcangio M, Strichartz GR. 2009; MAP kinase and pain. Brain Res Rev. 60:135–48. DOI: 10.1016/j.brainresrev.2008.12.011. PMID: 19150373. PMCID: PMC2666786.
Article37. Lisi L, Aceto P, Navarra P, Dello Russo C. 2015; mTOR kinase: a possible pharmacological target in the management of chronic pain. Biomed Res Int. 2015:394257. DOI: 10.1155/2015/394257. PMID: 25685786. PMCID: PMC4313067.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute effects of sodium salicylate on concentrations of catecholamine in the perilymph
- Effect of the combination of metformin and fenofibrate on glucose homeostasis in diabetic Goto-Kakizaki rats
- Sodium salicylate sensitivity in an asthmatic patient with aspirin sensitivity
- A Case of Bilateral Sudden Hearing Loss and Tinnitus after Salicylate Intoxication
- The Effect of Ototoxic Sodium Salicylate on DPOAE and Cochlear Blood Flow in Guinea Pig