Cancer Res Treat.
2024 Jul;56(3):920-935. 10.4143/crt.2023.869.
Feasibility of Circulating Tumor DNA Analysis in Patients with Follicular Lymphoma
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Geninus Inc., Seoul, Korea
- 3Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Health Sciences and Technology, Samsung Advanced Institute for Health Sciences and Technology, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2557679
- DOI: http://doi.org/10.4143/crt.2023.869
Abstract
- Purpose
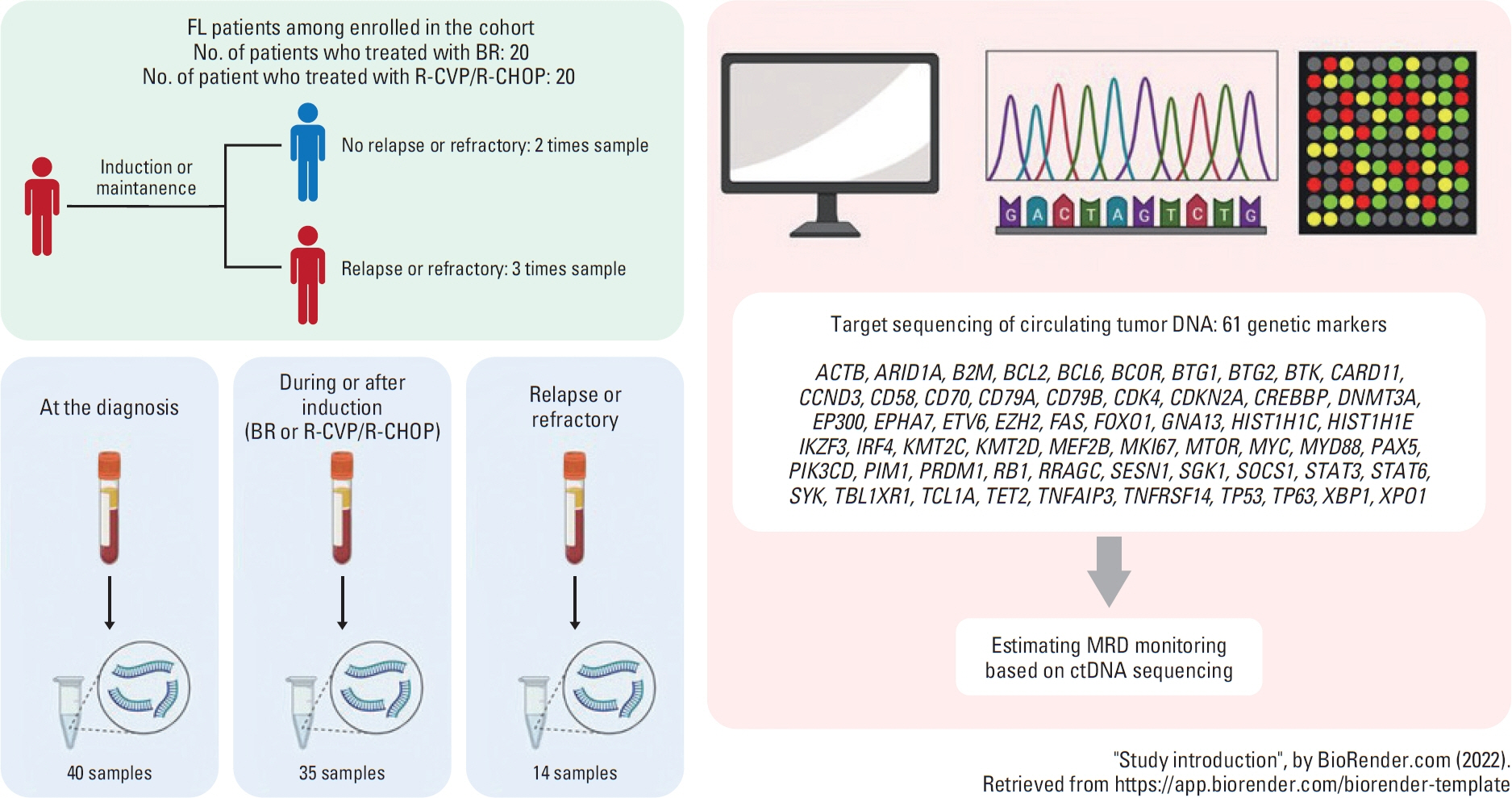
The feasibility of sequencing circulating tumor DNA (ctDNA) in plasma as a biomarker to predict early relapse or poor prognosis in patients with follicular lymphoma (FL) receiving systemic immunochemotherapy is not clear.
Materials and Methods
We sequenced DNA from cell-free plasma that was serially obtained from newly diagnosed FL patients undergoing systemic immunochemotherapy. The mutation profiles of ctDNA at the time of diagnosis and at response evaluation and relapse and/or progression were compared with clinical course and treatment outcomes.
Results
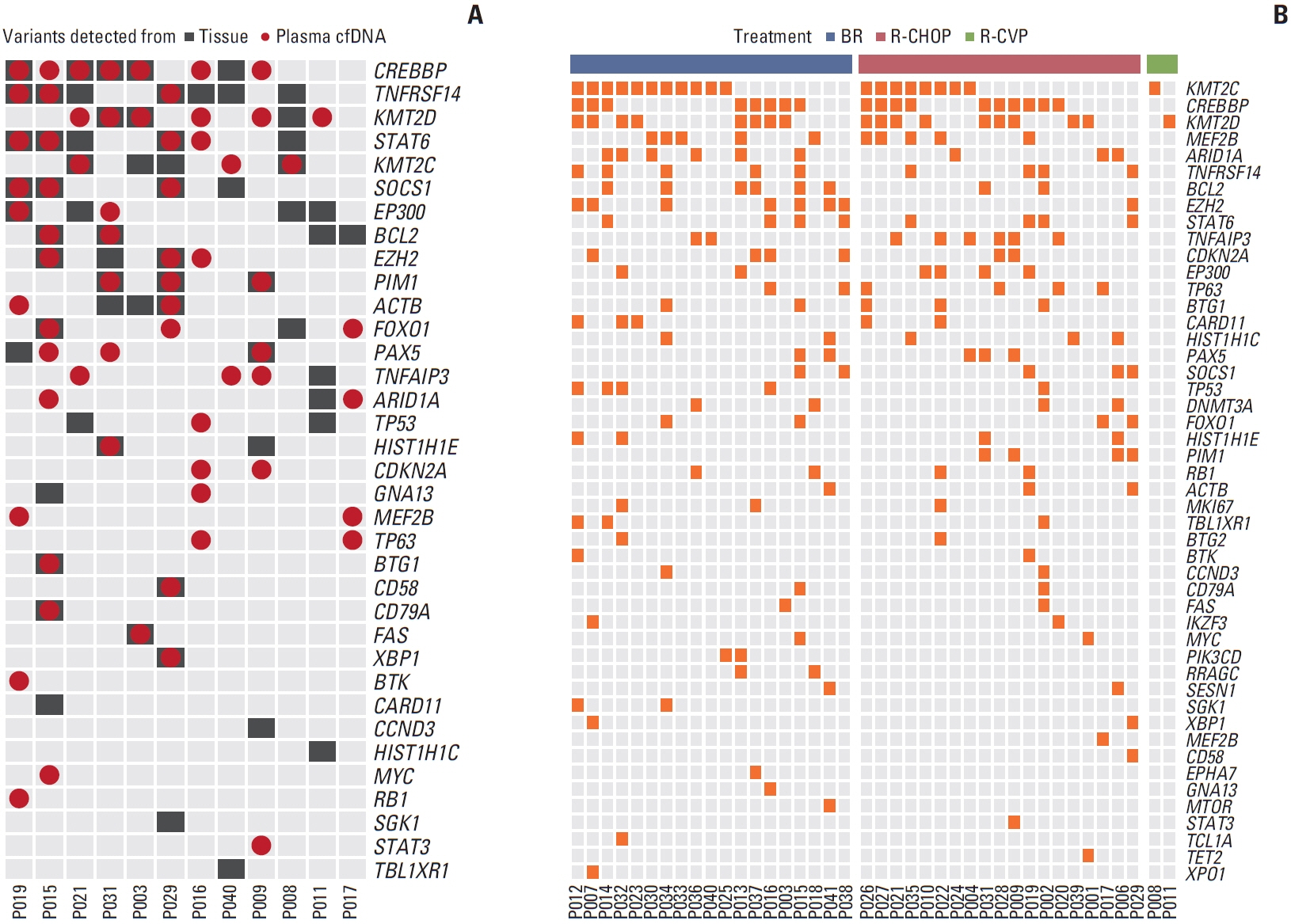
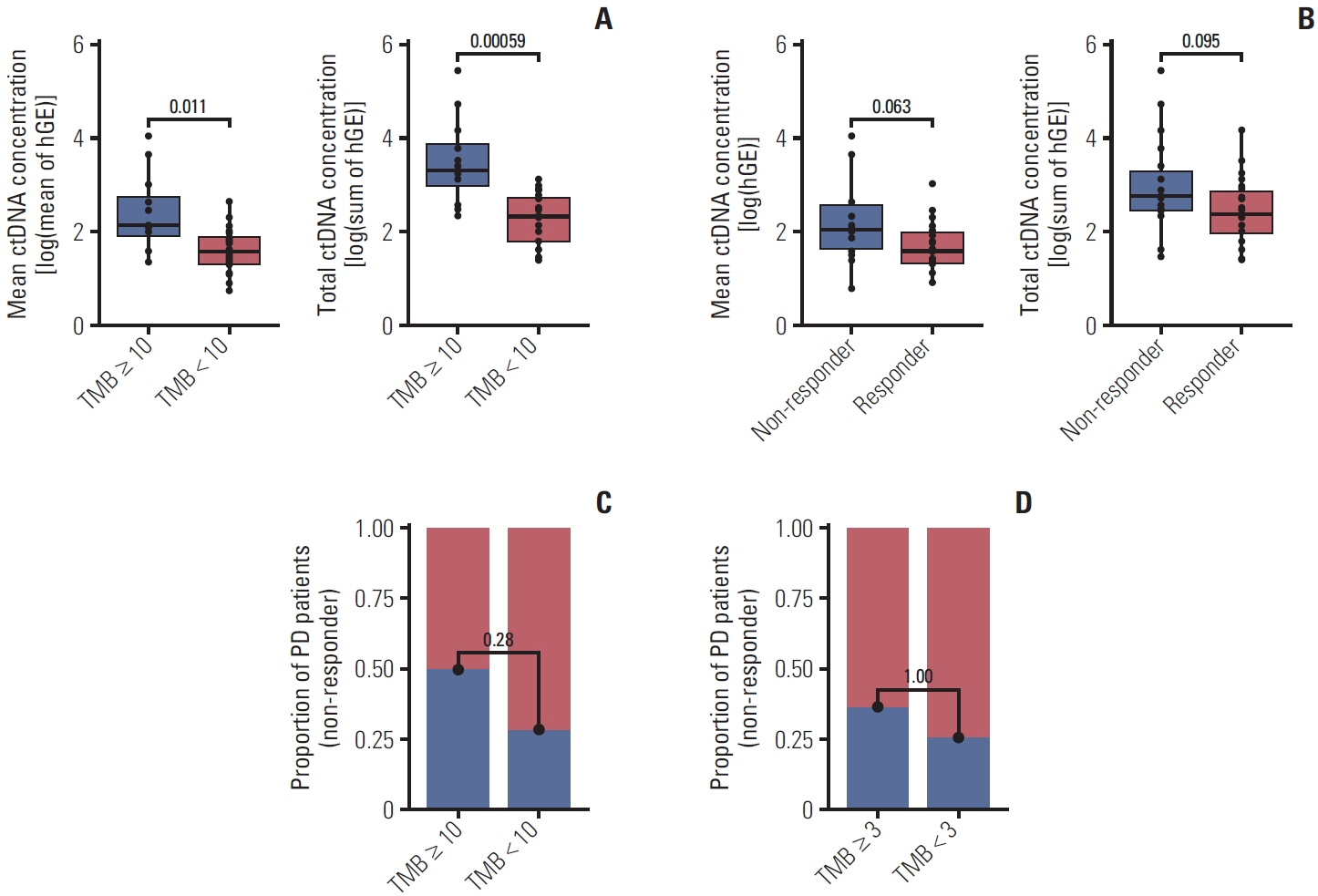
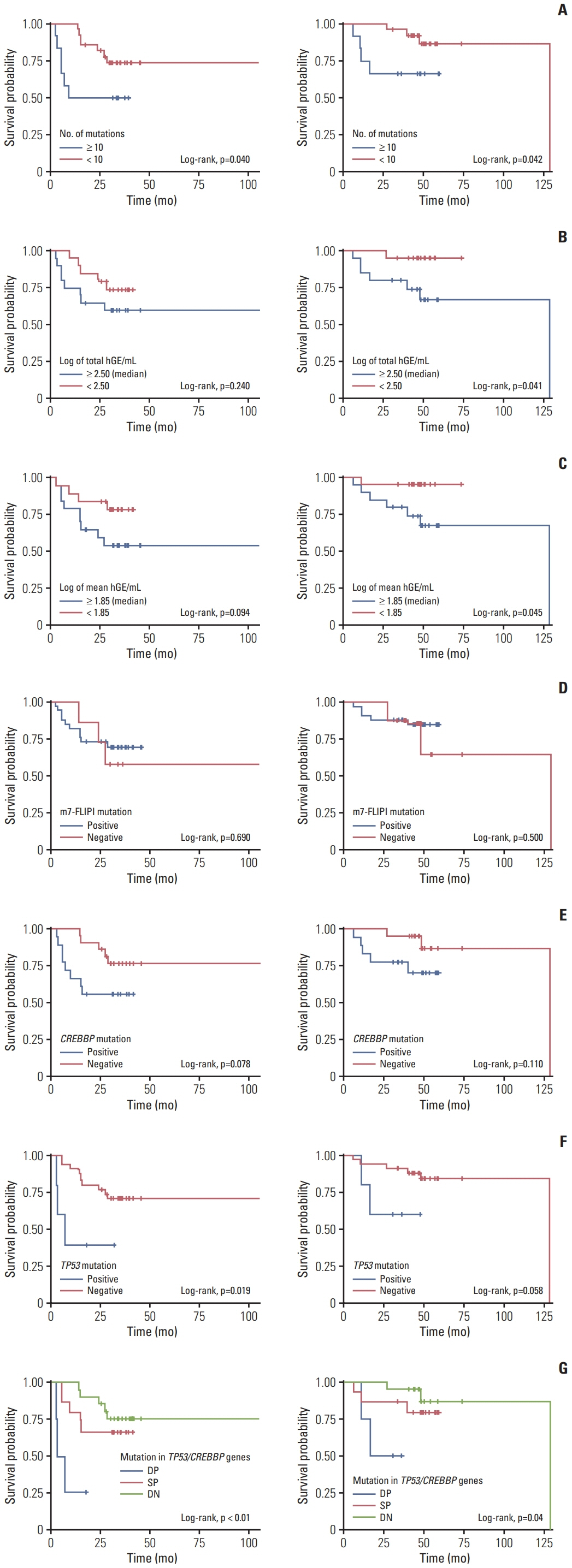
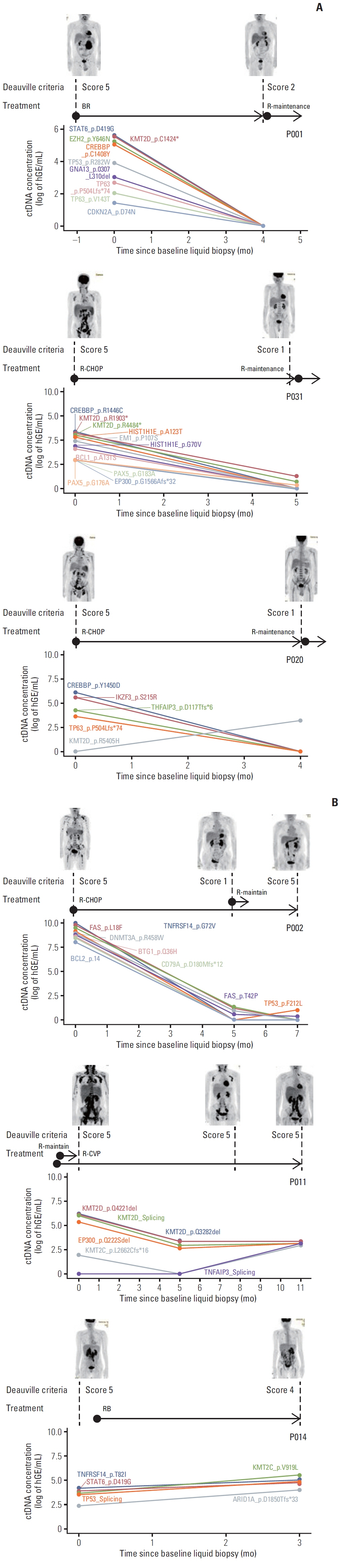
Forty samples from patients receiving rituximab-containing immunochemotherapy were analyzed. Baseline sequencing detected mutations in all cases, with the major detected mutations being KMT2C (50%), CREBBP (45%), and KMT2D (45%). The concentration of ctDNA and tumor mutation burden showed a significant association with survival outcome. In particular, the presence of mutations in CREBBP and TP53 showed poor prognosis compared with patients without them. Longitudinal analysis of ctDNA using serially collected plasma samples showed an association between persistence or reappearance of ctDNA mutations and disease relapse or progression.
Conclusion
Analysis of ctDNA mutations in plasma at diagnosis might help predict outcome of disease, while analysis during follow-up may help to monitor disease status of patients with advanced FL. However, the feasibility of ctDNA measurement must be improved in order for it to become an appropriate and clinically relevant test in FL patients.
Figure
Reference
-
References
1. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127:2375–90.
Article2. Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016; 66:443–59.
Article3. Dreyling M, Ghielmini M, Rule S, Salles G, Vitolo U, Ladetto M, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016; 27:v83–90.
Article4. Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005; 106:3725–32.
Article5. Marcus R, Imrie K, Solal-Celigny P, Catalano JV, Dmoszynska A, Raposo JC, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008; 26:4579–86.
Article6. Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grunhagen U, Losem C, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an openlabel, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013; 381:1203–10.7. Schulz H, Bohlius JF, Trelle S, Skoetz N, Reiser M, Kober T, et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2007; 99:706–14.
Article8. Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med. 2017; 377:1331–44.
Article9. Flinn IW, van der Jagt R, Kahl BS, Wood P, Hawkins TE, Macdonald D, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014; 123:2944–52.
Article10. Bachy E, Seymour JF, Feugier P, Offner F, Lopez-Guillermo A, Belada D, et al. Sustained progression-free survival benefit of rituximab maintenance in patients with follicular lymphoma: long-term results of the PRIMA study. J Clin Oncol. 2019; 37:2815–24.
Article11. Casulo C, Byrtek M, Dawson KL, Zhou X, Farber CM, Flowers CR, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol. 2015; 33:2516–22.
Article12. Seymour JF, Marcus R, Davies A, Gallop-Evans E, Grigg A, Haynes A, et al. Association of early disease progression and very poor survival in the GALLIUM study in follicular lymphoma: benefit of obinutuzumab in reducing the rate of early progression. Haematologica. 2019; 104:1202–8.
Article13. Wagner-Johnston ND, Link BK, Byrtek M, Dawson KL, Hainsworth J, Flowers CR, et al. Outcomes of transformed follicular lymphoma in the modern era: a report from the National LymphoCare Study (NLCS). Blood. 2015; 126:851–7.
Article14. Freeman CL, Kridel R, Moccia AA, Savage KJ, Villa DR, Scott DW, et al. Early progression after bendamustine-rituximab is associated with high risk of transformation in advanced stage follicular lymphoma. Blood. 2019; 134:761–4.
Article15. Yoon SE, Cho J, Kim WS, Kim SJ. Impact of transformation on the survival of patients diagnosed with follicular lymphoma that progressed within 24 months. J Cancer. 2021; 12:2488–97.
Article16. Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014; 32:579–86.
Article17. Camus V, Viennot M, Lequesne J, Viailly PJ, Bohers E, Bessi L, et al. Targeted genotyping of circulating tumor DNA for classical Hodgkin lymphoma monitoring: a prospective study. Haematologica. 2021; 106:154–62.
Article18. Lakhotia R, Melani C, Dunleavy K, Pittaluga S, Saba N, Lindenberg L, et al. Circulating tumor DNA predicts therapeutic outcome in mantle cell lymphoma. Blood Adv. 2022; 6:2667–80.
Article19. Herrera AF, Tracy S, Croft B, Opat S, Ray J, Lovejoy AF, et al. Risk profiling of patients with relapsed/refractory diffuse large B-cell lymphoma by measuring circulating tumor DNA. Blood Adv. 2022; 6:1651–60.
Article20. Freedman A, Jacobsen E. Follicular lymphoma: 2020 update on diagnosis and management. Am J Hematol. 2020; 95:316–27.
Article21. Solal-Celigny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, et al. Follicular lymphoma international prognostic index. Blood. 2004; 104:1258–65.
Article22. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32:3059–68.
Article23. Tobin JW, Keane C, Gunawardana J, Mollee P, Birch S, Hoang T, et al. Progression of disease within 24 months in follicular lymphoma is associated with reduced intratumoral immune infiltration. J Clin Oncol. 2019; 37:3300–9.
Article24. Pastore A, Jurinovic V, Kridel R, Hoster E, Staiger AM, Szczepanowski M, et al. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol. 2015; 16:1111–22.
Article25. Carbone A, Roulland S, Gloghini A, Younes A, von Keudell G, Lopez-Guillermo A, et al. Follicular lymphoma. Nat Rev Dis Primers. 2019; 5:83.
Article26. Green MR. Chromatin modifying gene mutations in follicular lymphoma. Blood. 2018; 131:595–604.
Article27. O’Shea D, O’Riain C, Taylor C, Waters R, Carlotti E, Macdougall F, et al. The presence of TP53 mutation at diagnosis of follicular lymphoma identifies a high-risk group of patients with shortened time to disease progression and poorer overall survival. Blood. 2008; 112:3126–9.
Article28. McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010; 20:1297–303.
Article29. Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011; 27:2987–93.
Article30. Newman AM, Lovejoy AF, Klass DM, Kurtz DM, Chabon JJ, Scherer F, et al. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat Biotechnol. 2016; 34:547–55.
Article31. McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GR, Thormann A, et al. The Ensembl variant effect predictor. Genome Biol. 2016; 17:122.32. Avanzini S, Kurtz DM, Chabon JJ, Moding EJ, Hori SS, Gambhir SS, et al. A mathematical model of ctDNA shedding predicts tumor detection size. Sci Adv. 2020; 6:eabc4308.
Article33. Cho J, Yoon SE, Kim SJ, Ko YH, Kim WS. Comparison of tumor mutation burden of 300 various non-Hodgkin lymphomas using panel based massively parallel sequencing. BMC Cancer. 2021; 21:972.
Article34. Shin SH, Kim YJ, Lee D, Cho D, Ko YH, Cho J, et al. Analysis of circulating tumor DNA by targeted ultra-deep sequencing across various non-Hodgkin lymphoma subtypes. Leuk Lymphoma. 2019; 60:2237–46.
Article35. Yoon SE, Kim YJ, Shim JH, Park D, Cho J, Ko YH, et al. Plasma circulating tumor DNA in patients with primary central nervous system lymphoma. Cancer Res Treat. 2022; 54:597–612.
Article36. Hur JY, Kim YJ, Yoon SE, Son DS, Park WY, Kim SJ, et al. Plasma cell-free DNA is a prognostic biomarker for survival in patients with aggressive non-Hodgkin lymphomas. Ann Hematol. 2020; 99:1293–302.
Article37. Kim SJ, Kim YJ, Yoon SE, Ryu KJ, Park B, Park D, et al. Circulating tumor DNA-based genotyping and monitoring for predicting disease relapses of patients with peripheral T-cell lymphomas. Cancer Res Treat. 2023; 55:291–303.
Article38. Kim JJ, Kim HY, Choi Z, Hwang SY, Jeong H, Choi JR, et al. In-depth circulating tumor DNA sequencing for prognostication and monitoring in natural killer/T-cell lymphomas. Front Oncol. 2023; 13:1109715.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Application of Circulating Tumor DNA Analysis
- Dermatomyositis Associated with Follicular Lymphoma
- Detection of bcl-2/IgH Gene Rearrangement and Expression of c-myc and p53 Oncoprotein in B-cell Lymphoma
- Composite follicular lymphoma and classic Hodgkin lymphoma
- Circulating Lymphoma Cells in the Peripheral Blood from 4 Cases of Mantle and T Cell Types of Non-Hodgkin's Lymphoma: Light and Electron Microscopic Morphology