Cancer Res Treat.
2024 Jul;56(3):909-919. 10.4143/crt.2023.1174.
The Role of Early and Delayed Surgery for Infants with Congenital Brain Tumors
- Affiliations
-
- 1Division of Pediatric Neurosurgery, Seoul National University Children’s Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Neural Development and Anomaly Laboratory, Department of Anatomy and Cell Biology, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Pathology, Seoul National University Children’s Hospital, Seoul National University College of Medicine, Seoul, Korea
- 4Neuroscience Research Institute, Seoul National University Medical Research Center, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2557678
- DOI: http://doi.org/10.4143/crt.2023.1174
Abstract
- Purpose
The present study aimed to evaluate the role of early and delayed surgery in congenital brain tumors and analyze the clinical outcomes of infantile brain tumors.
Materials and Methods
We performed a retrospective cohort study on 69 infantile brain tumors at a single institution from January 2008 to June 2023. Outcomes were assessed as early mortality (within 30 days following surgery) to evaluate the risk of early surgery in congenital brain tumors. Outcomes of recurrence and overall survival were analyzed in infantile brain tumors.
Results
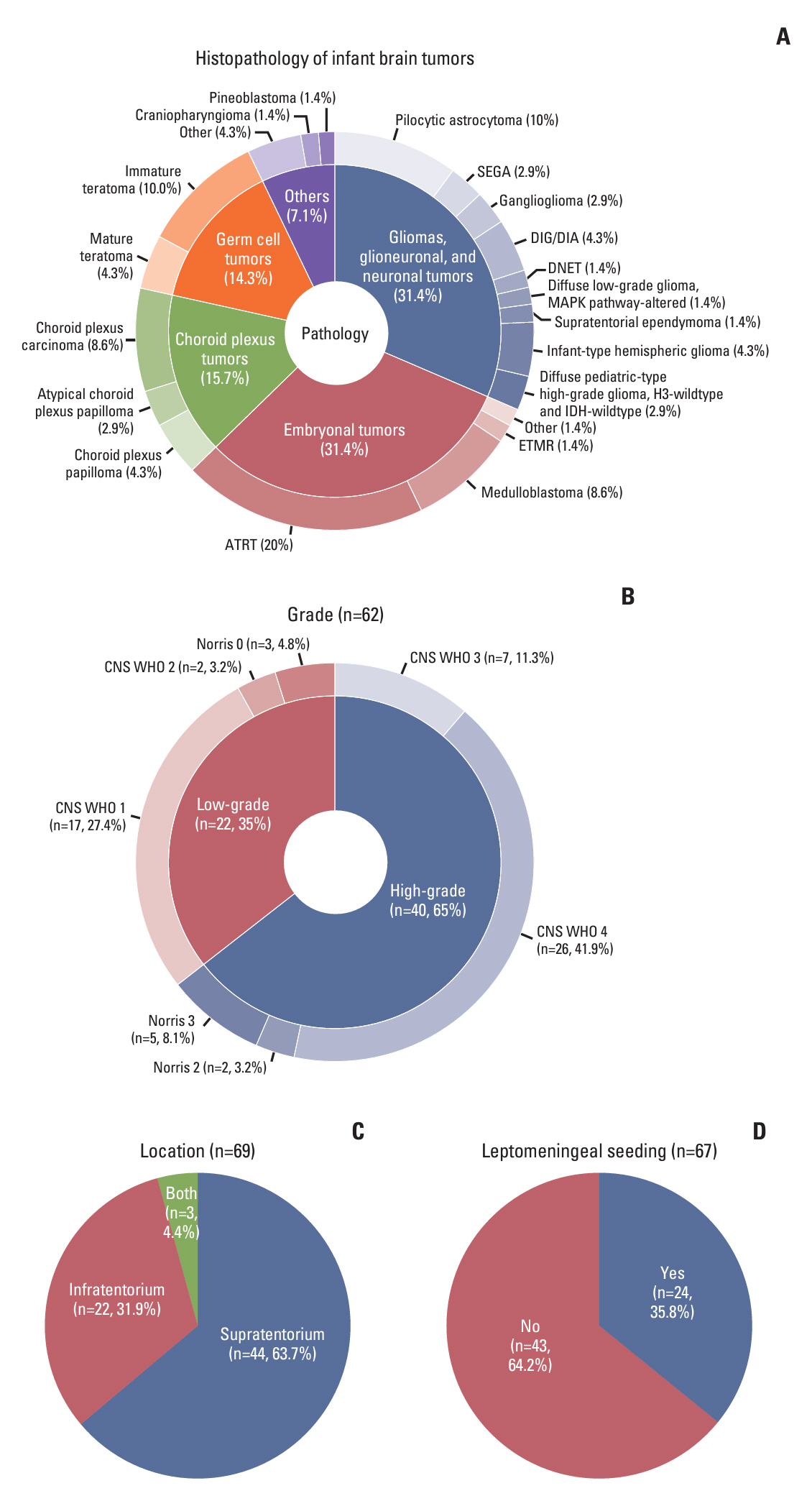
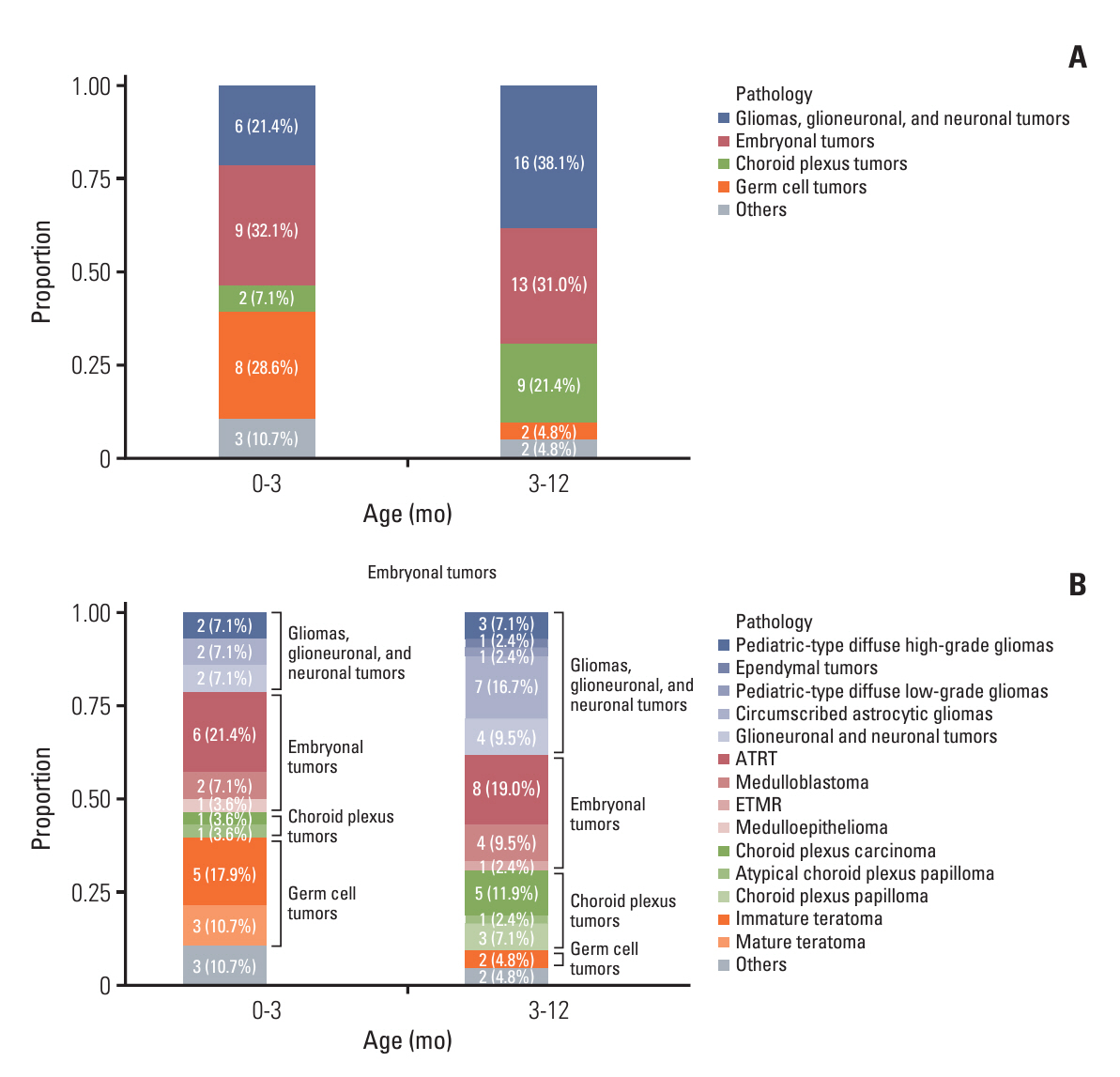
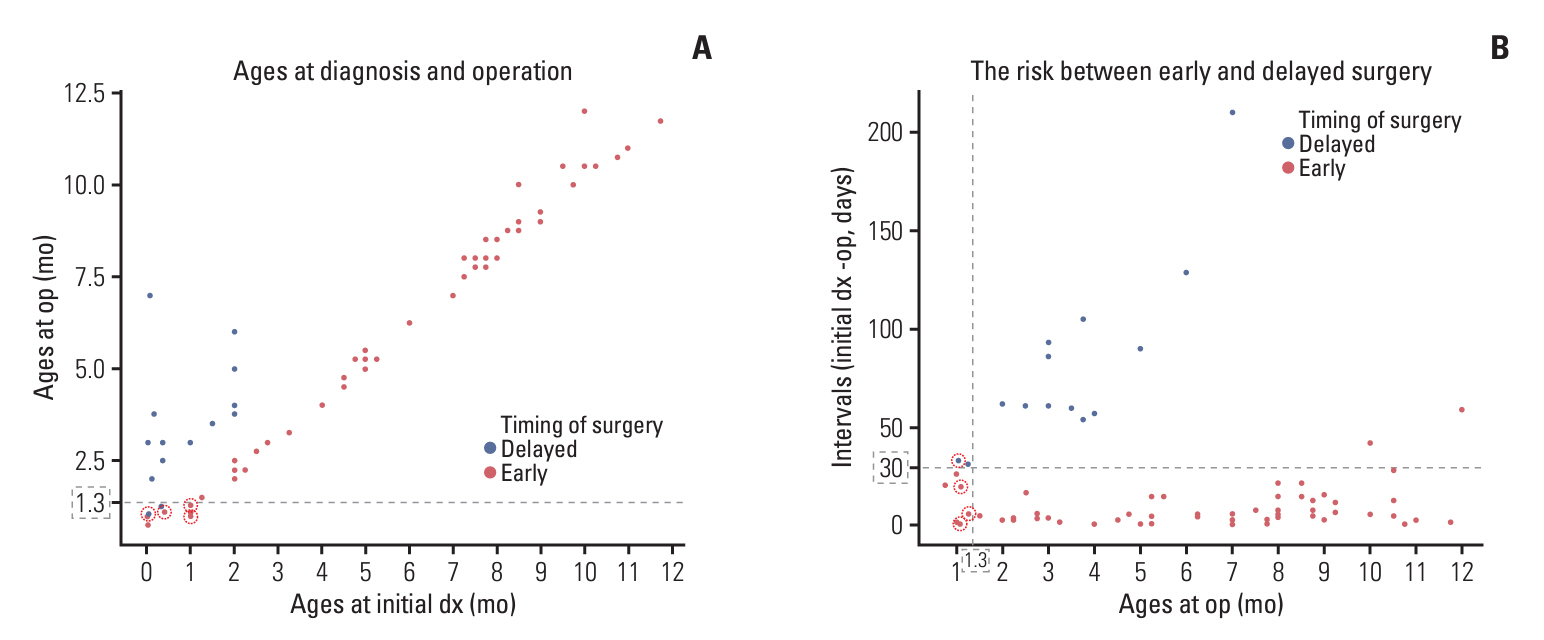
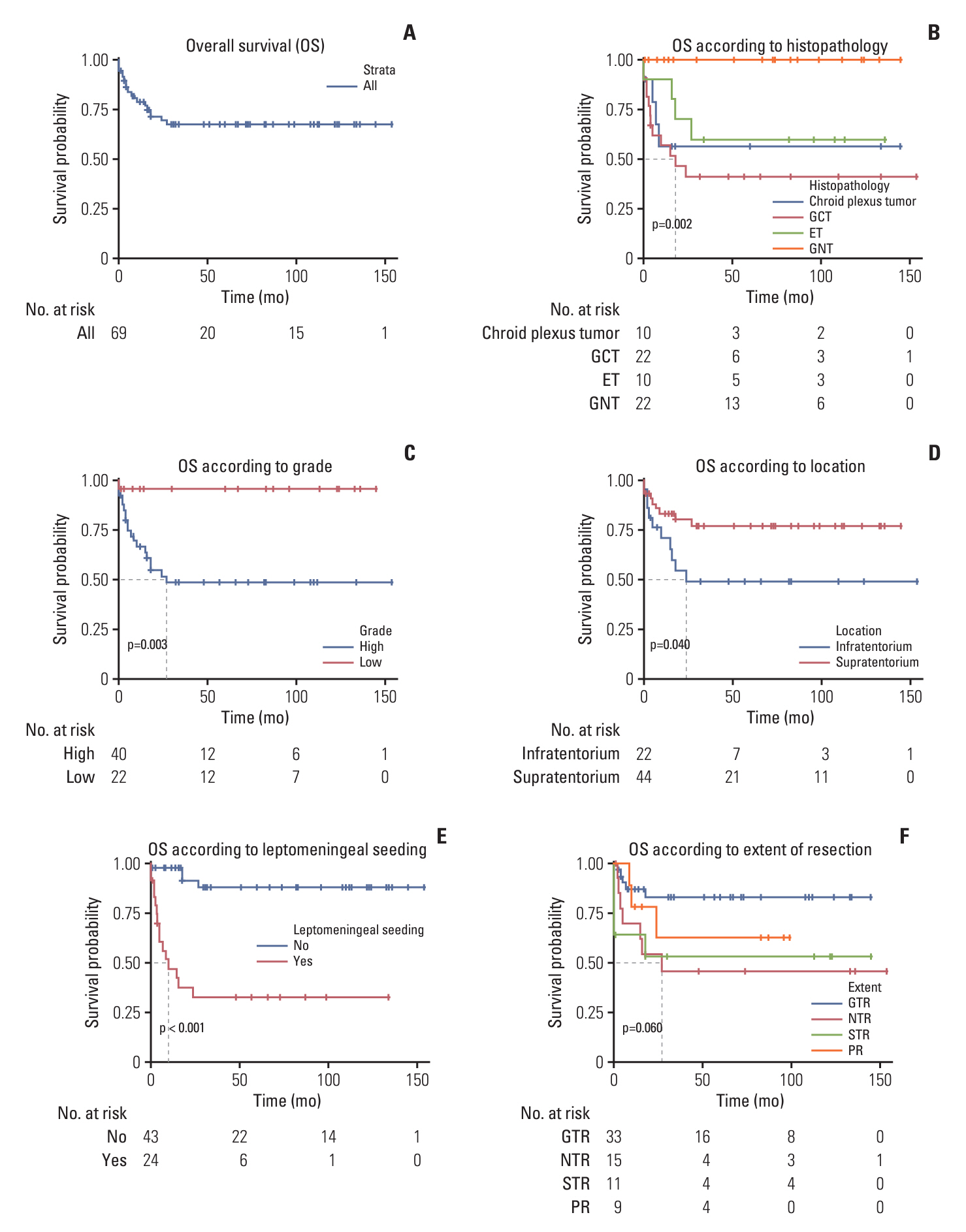
Surgery-related early mortality appeared to occur in young and low-body-weight patients. Cut-off values of age and body weight were found to be 1.3 months and 5.2 kg to avoid early mortality. Three patients (3/10, 30%) showed early mortality in the early surgery group, and early mortality occurred in one patient (1/14, 7.14%) in the delayed surgery group, whose tumor was excessively enlarged. Younger age at diagnosis (< 3 months of age; hazard ratios [HR], 7.1; 95% confidence intervals [CI], 1.4 to 35.6; p=0.018) and leptomeningeal seeding (LMS; HR, 30.6; 95% CI, 3.7 to 253.1; p=0.002) were significant independent risk factors for high mortality in infantile brain tumors.
Conclusion
We suggest delaying surgery until the patient reaches 1.3 months of age and weighs over 5.2 kg with short-term imaging follow-up unless tumors grow rapidly in congenital brain tumors. Younger ages and the presence of LMS are independent risk factors for high mortality in infantile brain tumors.
Keyword
Figure
Reference
-
References
1. Lamba N, Groves A, Torre M, Yeo KK, Iorgulescu JB. The epidemiology of primary and metastatic brain tumors in infancy through childhood. J Neurooncol. 2022; 156:419–29.
Article2. Shekdar KV, Schwartz ES. Brain tumors in the neonate. Neuroimaging Clin N Am. 2017; 27:69–83.3. Buetow PC, Smirniotopoulos JG, Done S. Congenital brain tumors: a review of 45 cases. AJR Am J Roentgenol. 1990; 155:587–93.
Article4. Carstensen H, Juhler M, Bogeskov L, Laursen H. A report of nine newborns with congenital brain tumours. Childs Nerv Syst. 2006; 22:1427–31.
Article5. Jurkiewicz E, Brozyna A, Grajkowska W, Bekiesinska-Figatowska M, Daszkiewicz P, Nowak K, et al. Congenital brain tumors in a series of 56 patients. Childs Nerv Syst. 2012; 28:1193–201.
Article6. Manoranjan B, Provias JP. Congenital brain tumors: diagnostic pitfalls and therapeutic interventions. J Child Neurol. 2011; 26:599–614.
Article7. Hart M, Anderson-Mellies A, Beltrami A, Gilani A, Green AL. Population-based analysis of CNS tumor diagnoses, treatment, and survival in congenital and infant age groups. J Neurooncol. 2022; 157:333–44.8. Viaene AN, Pu C, Perry A, Li MM, Luo M, Santi M. Congenital tumors of the central nervous system: an institutional review of 64 cases with emphasis on tumors with unique histologic and molecular characteristics. Brain Pathol. 2021; 31:45–60.
Article9. Lasky JL, Choi EJ, Johnston S, Yong WH, Lazareff J, Moore T. Congenital brain tumors: case series and review of the literature. J Pediatr Hematol Oncol. 2008; 30:326–31.10. Mazewski CM, Hudgins RJ, Reisner A, Geyer JR. Neonatal brain tumors: a review. Semin Perinatol. 1999; 23:286–98.
Article11. Mohanty CB, Shukla DP, Devi BI. Brain tumors of infancy: an institutional experience and review of the literature. Pediatr Neurosurg. 2013; 49:145–54.
Article12. Anderson BR, Blancha Eckels VL, Crook S, Duchon JM, Kalfa D, Bacha EA, et al. The risks of being tiny: the added risk of low weight for neonates undergoing congenital heart surgery. Pediatr Cardiol. 2020; 41:1623–31.13. Mehmood A, Ismail SR, Kabbani MS, Abu-Sulaiman RM, Najm HK. Outcome of low body weight (< 2.2 kg) infants undergoing cardiac surgery. J Saudi Heart Assoc. 2014; 26:132–7.14. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021; 23:1231–51.
Article15. Norris HJ, Zirkin HJ, Benson WL. Immature (malignant) teratoma of the ovary: a clinical and pathologic study of 58 cases. Cancer. 1976; 37:2359–72.
Article16. O’Connor DM, Norris HJ. The influence of grade on the outcome of stage I ovarian immature (malignant) teratomas and the reproducibility of grading. Int J Gynecol Pathol. 1994; 13:283–9.
Article17. Phi JH, Kim SK, Park SH, Hong SH, Wang KC, Cho BK. Immature teratomas of the central nervous system: is adjuvant therapy mandatory? J Neurosurg. 2005; 103:524–30.18. Hardesty DA, Sanai N. The value of glioma extent of resection in the modern neurosurgical era. Front Neurol. 2012; 3:140.
Article19. Joo JD, Kim H, Kim YH, Han JH, Kim CY. Validation of the effectiveness and safety of temozolomide during and after radiotherapy for newly diagnosed glioblastomas: 10-year experience of a single institution. J Korean Med Sci. 2015; 30:1597–603.
Article20. Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol. 2022; 24:v1–95.
Article21. Ostrom QT, Price M, Ryan K, Edelson J, Neff C, Cioffi G, et al. CBTRUS statistical report: pediatric brain tumor foundation childhood and adolescent primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol. 2022; 24:iii1–38.
Article22. Gianno F, Giovannoni I, Cafferata B, Diomedi-Camassei F, Minasi S, Barresi S, et al. Paediatric-type diffuse high-grade gliomas in the 5th CNS WHO Classification. Pathologica. 2022; 114:422–35.23. Kaatsch P, Hafner C, Calaminus G, Blettner M, Tulla M. Pediatric germ cell tumors from 1987 to 2011: incidence rates, time trends, and survival. Pediatrics. 2015; 135:e136–43.
Article24. McCarthy BJ, Shibui S, Kayama T, Miyaoka E, Narita Y, Murakami M, et al. Primary CNS germ cell tumors in Japan and the United States: an analysis of 4 tumor registries. Neuro Oncol. 2012; 14:1194–200.
Article25. Oosterhuis JW, Stoop H, Honecker F, Looijenga LH. Why human extragonadal germ cell tumours occur in the midline of the body: old concepts, new perspectives. Int J Androl. 2007; 30:256–63.
Article26. Han F, Wang Y, Wang Y, Dong J, Nie C, Chen M, et al. Intraoperative cardiac arrest: A 10-year study of patients undergoing tumorous surgery in a tertiary referral cancer center in China. Medicine (Baltimore). 2017; 96:e6794.27. Riley AA, Arakawa Y, Worley S, Duncan BW, Fukamachi K. Circulating blood volumes: a review of measurement techniques and a meta-analysis in children. ASAIO J. 2010; 56:260–4.28. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018; 61:135–49.
Article29. Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999; 25:103–19.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nutrition of the Infants and Children with Cardiac Disease
- Congenital Syphilis: Hematologic Findings of Early Congenital Syphilis
- Brain tumor in the first year of life: a single institute study
- Rapid Progression of Early Delayed Radiation Effect in Pleomorphic Xanthoastrocytoma
- Concept of developmental disability and the role of a pediatrician