Cancer Res Treat.
2024 Jul;56(3):898-908. 10.4143/crt.2023.981.
Predicted Cervical Cancer Prevention: Impact of National HPV Vaccination Program on Young Women in South Korea
- Affiliations
-
- 1Department of Occupational and Environmental Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea
- 2Department of Environmental and Occupational Health, Korea University Graduate School of Public Health, Seoul, Korea
- 3Department of Public Health Sciences, Seoul National University Graduate School of Public Health, Seoul, Korea
- KMID: 2557677
- DOI: http://doi.org/10.4143/crt.2023.981
Abstract
- Purpose
This study aimed to evaluate the effectiveness of the national human papillomavirus (HPV) vaccination program of South Korea among its entire female population, particularly among younger age groups.
Materials and Methods
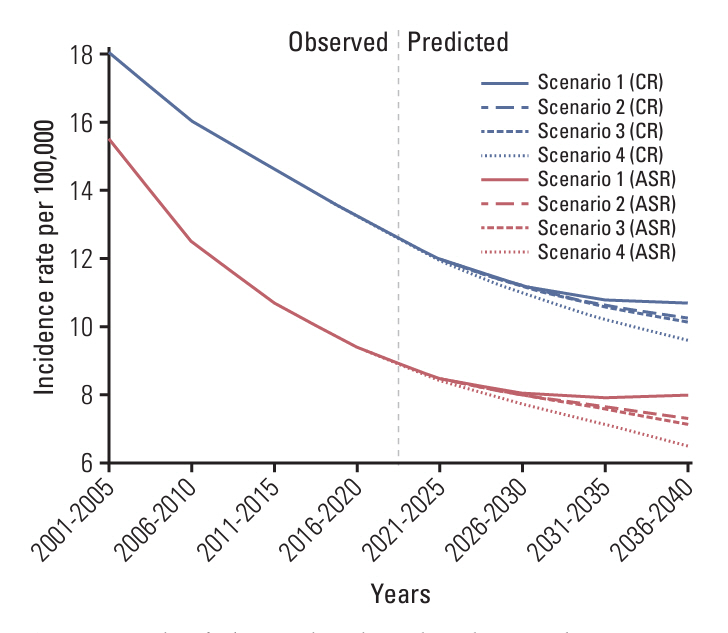
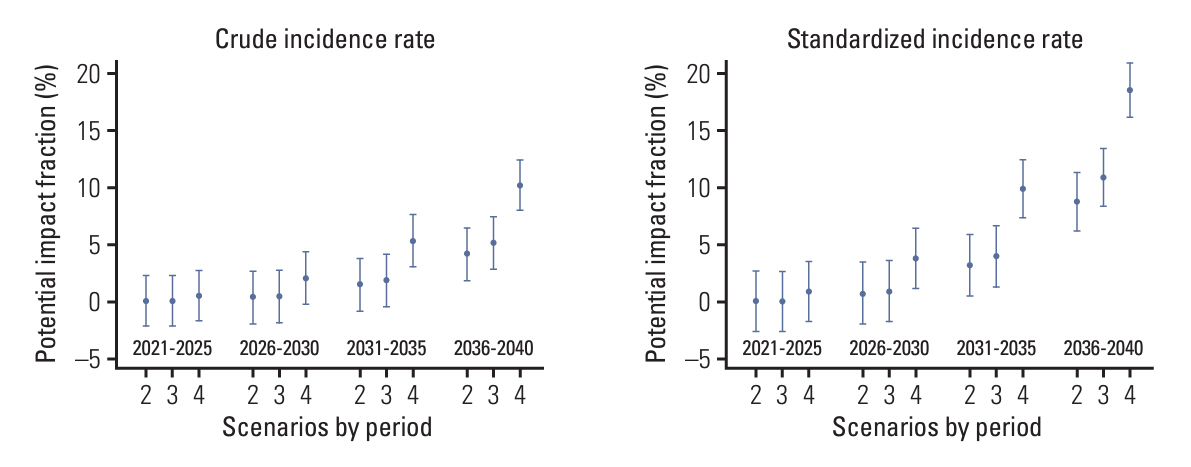
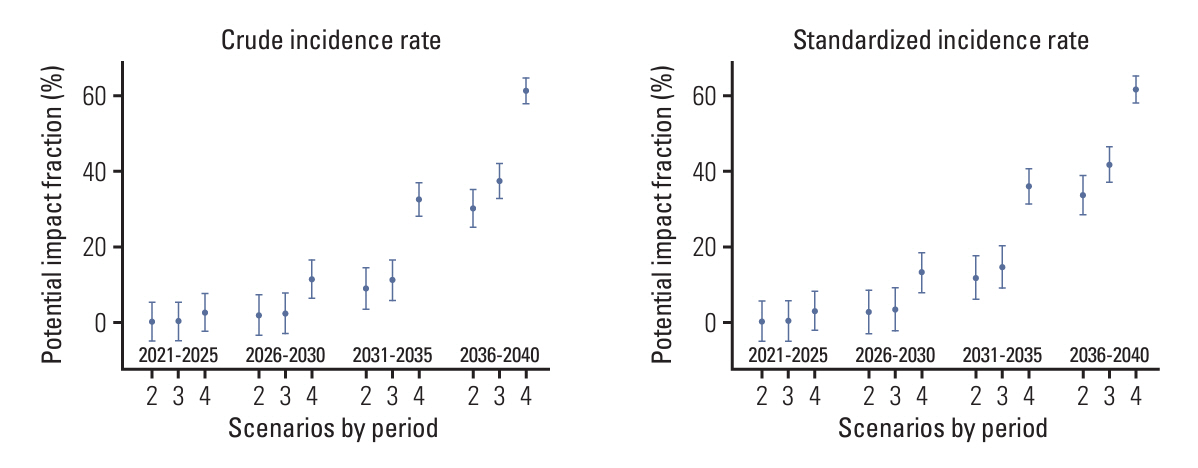
We first predicted the incidence of cervical cancer over the next 20 years (2021-2040) using the Nordpred package based on Møller’s age-period-cohort model under several scenarios for the national HPV vaccination program. We calculated the potential impact fractions and proportional differences under the current national vaccination programs, and alternative scenarios using the no-vaccination assumption as a reference.
Results
We estimated that the current national vaccination program would prevent 4.13% of cervical cancer cases and reduce the age-standardized incidence rate (ASR) by 8.79% in the overall population by 2036-2040. Under the alternative scenario of implementing the nine-valent vaccine, 5.13% of cervical cancer cases could be prevented and the ASR reduced by 10.93% during the same period. In another scenario, expanding the vaccination age to 9-17 years could prevent 10.19% of cervical cancer cases, with the ASR reduced by 18.57% during the same period. When restricted to ages < 40 years, the prevention effect was remarkably greater. We predict that the current national HPV program will reduce its incidence by more than 30% between 2036 and 2040 in women aged < 40 years.
Conclusion
The effectiveness of the vaccination program in reducing the incidence of cervical cancer was confirmed, with a considerable impact anticipated in younger age groups.
Keyword
Figure
Reference
-
References
1. National Cancer Institution. Cervical cancer treatment [Internet]. Bethesda, MD: National Cancer Institution;c2022. [cited 2022 Sep 12]. Available from: https://www.cancer.gov/types/cervical/patient/cervical-treatment-pdq#section/all.2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.
Article3. Korea Central Cancer Registry; National Cancer Center. Annual report of cancer statistics in Korea in 2020: National Cancer Control Project [Internet]. Goyang: National Cancer Cancer;c2022. [cited 2023 Mar 12]. Available from: http://ncc.re.kr/cancerStatsList.ncc?searchKey=total&searchValue=&pageNum=1.4. Korean Statistical Information Service. Cause of death statistics [Internet]. Daejeon: Statistics Korea;c2023. [cited 2023 Mar 12]. Available from: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1B34E09&vw_cd=MT_ZTITLZ&list_id=F_27&seqNo=&lang_mode=ko&language=kor&obj_var_id=&itm_id=&conn_papa=MT_ZTITLE.5. Castellsague X, Bosch FX, Munoz N, Meijer CJ, Shah KV, de Sanjose S, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002; 346:1105–12.
Article6. Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995; 87:796–802.
Article7. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999; 189:12–9.
Article8. Stewart BW, Wild CP. World cancer report 2014. Chapter 5.12. Cancers of the female reproductive organ. Geneva: World Health Organization;2014. p. 465–82.9. Kessler TA. Cervical cancer: prevention and early detection. Semin Oncol Nurs. 2017; 33:172–83.10. Munoz N, Castellsague X, Berrington de Gonzalez A, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006; 24 Suppl 3:S3/1–10.
Article11. National Cancer Institute. Cervical cancer prevention (PDQ): health professional version [Internet]. Bethesda, MD: National Cancer Institute;c2023. [cited 2023 Apr 10]. Available from: https://www.cancer.gov/types/cervical/hp/cervical-prevention-pdq.12. World Health Organization. Electronic address swi. Human papillomavirus vaccines: WHO position paper, May 2017-Recommendations. Vaccine. 2017; 35:5753–5.13. World Health Organization. Cervical cancer [Internet]. Geneva: World Health Organization;c2022. [cited 2022 Oct 8]. Available from: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer.14. Gearhart PA, Randall TC, Buckley RM Jr. Human papillomavirus (HPV) clinical presentation [Internet]. New York: Medscape;c2020. [cited 2022 Dec 8]. Available from: https://emedicine.medscape.com/article/219110-clinical.15. Petrosky E, Bocchini JA Jr, Hariri S, Chesson H, Curtis CR, Saraiya M, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015; 64:300–4.16. Meeting of the Strategic Advisory Group of Experts on immunization, April 2014: conclusions and recommendations. Wkly Epidemiol Rec. 2014; 89:221–36.17. Kreimer AR, Sampson JN, Porras C, Schiller JT, Kemp T, Herrero R, et al. Evaluation of durability of a single dose of the bivalent HPV vaccine: the CVT trial. J Natl Cancer Inst. 2020; 112:1038–46.
Article18. Basu P, Malvi SG, Joshi S, Bhatla N, Muwonge R, Lucas E, et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study. Lancet Oncol. 2021; 22:1518–29.
Article19. Barnabas RV, Brown ER, Onono MA, Bukusi EA, Njoroge B, Winer RL, et al. Efficacy of single-dose HPV vaccination among young African women. NEJM Evid. 2022; 1:EVIDoa2100056.20. World Health Organization. Meeting of the strategic advisory group of experts on immunization, April 2022: conclusions and recommendations. Wkly Epidemiol Rec. 2022; 97:261–76.21. Shin HR, Won YJ, Jung KW, Kong HJ, Yim SH, Lee JK, et al. Nationwide cancer incidence in Korea, 1999~2001; first result using the national cancer incidence database. Cancer Res Treat. 2005; 37:325–31.
Article22. Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016; 48:436–50.
Article23. Berman TA, Schiller JT. Human papillomavirus in cervical cancer and oropharyngeal cancer: one cause, two diseases. Cancer. 2017; 123:2219–29.
Article24. Kim YT, Serrano B, Lee JK, Lee H, Lee SW, Freeman C, et al. Burden of Human papillomavirus (HPV)-related disease and potential impact of HPV vaccines in the Republic of Korea. Papillomavirus Res. 2019; 7:26–42.
Article25. Arbyn M, Xu L. Efficacy and safety of prophylactic HPV vaccines. A Cochrane review of randomized trials. Expert Rev Vaccines. 2018; 17:1085–91.
Article26. Centers for Disease Control and Prevention. Human papillomavirus (HPV): vaccine information for young women [Internet]. Atlanta, GA: Centers for Disease Control and Prevention;c2022. [cited 2022 Dec 20]. Available from: https://www.cdc.gov/std/hpv/stdfact-hpv-vaccine-young-women.htm.27. Antoni S, Soerjomataram I, Moller B, Bray F, Ferlay J. An assessment of GLOBOCAN methods for deriving national estimates of cancer incidence. Bull World Health Organ. 2016; 94:174–84.28. Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJ, Lozano R, Inoue M. Age standardization of rates: a new WHO standard. GPE Discussion Paper Series, No. 31. EIP/GPE/EBD. Geneva: World Health Organization;2001.29. Hildebrandt M, Bender R, Gehrmann U, Blettner M. Calculating confidence intervals for impact numbers. BMC Med Res Methodol. 2006; 6:32.
Article30. Olsen CM, Wilson LF, Green AC, Biswas N, Loyalka J, Whiteman DC. How many melanomas might be prevented if more people applied sunscreen regularly? Br J Dermatol. 2018; 178:140–7.
Article31. Ren X, Qiu L, Ke W, Zou H, Liu A, Wu T. Awareness and acceptance of HPV vaccination for condyloma acuminata among men who have sex with men in China. Hum Vaccin Immunother. 2022; 18:2115267.
Article32. World Health Organization. Vaccine in national immunization programme update [Internet]. Geneva: World Health Organization;c2020. [cited 2022 Oct 4]. Available from: https://cdn.who.int/media/docs/default-source/immunization/hpv/vaccineintrostatus.pdf?sfvrsn=a705e49c_.33. Gertig DM, Brotherton JM, Budd AC, Drennan K, Chappell G, Saville AM. Impact of a population-based HPV vaccination program on cervical abnormalities: a data linkage study. BMC Med. 2013; 11:227.
Article34. Powell SE, Hariri S, Steinau M, Bauer HM, Bennett NM, Bloch KC, et al. Impact of human papillomavirus (HPV) vaccination on HPV 16/18-related prevalence in precancerous cervical lesions. Vaccine. 2012; 31:109–13.
Article35. Cuschieri K, Kavanagh K, Cameron R, Bhatia R, Pollock KG. The massive decline of clinically relevant high-risk human papillomavirus (HR-HPV) infection in Scotland. In : Microbiology Society Annual Conference; 2017 Apr 3-6; Edinburgh, UK.36. Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Liu B, Bateson D, et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis. 2014; 14:958–66.
Article37. Ekwunife OI, O’Mahony JF, Gerber Grote A, Mosch C, Paeck T, Lhachimi SK. Challenges in cost-effectiveness analysis modelling of HPV vaccines in low- and middle-income countries: a systematic review and practice recommendations. Pharmacoeconomics. 2017; 35:65–82.
Article38. World Health Organization (WHO). Global strategy to accelerate the elimination of cervical cancer as a public health problem [Internet]. Geneva: World Health Organization;c2020. [cited 2023 Apr 4]. Available from: https://www.who.int/publications/i/item/9789240014107.39. Brotherton JM, Tabrizi SN, Phillips S, Pyman J, Cornall AM, Lambie N, et al. Looking beyond human papillomavirus (HPV) genotype 16 and 18: defining HPV genotype distribution in cervical cancers in Australia prior to vaccination. Int J Cancer. 2017; 141:1576–84.
Article40. Wnukowski-Mtonga P, Jayasinghe S, Chiu C, Macartney K, Brotherton J, Donovan B, et al. Scientific evidence supporting recommendations on the use of the 9-valent HPV vaccine in a 2-dose vaccine schedule in Australia. Commun Dis Intell (2018). 2020; 44:1–12.
Article41. Phillips A, Patel C, Pillsbury A, Brotherton J, Macartney K. Safety of human papillomavirus vaccines: an updated review. Drug Saf. 2018; 41:329–46.
Article42. Garland SM. The Australian experience with the human papillomavirus vaccine. Clin Ther. 2014; 36:17–23.
Article43. Sinka K, Kavanagh K, Gordon R, Love J, Potts A, Donaghy M, et al. Achieving high and equitable coverage of adolescent HPV vaccine in Scotland. J Epidemiol Community Health. 2014; 68:57–63.
Article44. Chido-Amajuoyi OG, Talluri R, Wonodi C, Shete S. Trends in HPV vaccination initiation and completion within ages 9-12 years: 2008-2018. Pediatrics. 2021; 147:e2020012765.45. Toh ZQ, Russell FM, Reyburn R, Fong J, Tuivaga E, Ratu T, et al. Sustained antibody responses 6 years following 1, 2, or 3 doses of quadrivalent human papillomavirus (HPV) vaccine in adolescent Fijian girls, and subsequent responses to a single dose of bivalent HPV vaccine: a prospective cohort study. Clin Infect Dis. 2017; 64:852–9.46. Lee EH, Um TH, Chi HS, Hong YJ, Cha YJ. Prevalence and distribution of human papillomavirus infection in Korean women as determined by restriction fragment mass polymorphism assay. J Korean Med Sci. 2012; 27:1091–7.
Article47. Lee SY, Lee JE, Gi MG, Kang C. An overview of immunization and efficacy of human papillomavirus vaccines. Public Health Wkly Rep. 2014; 7:1162–6.48. Comprehensive cervical cancer control: a guide to essential practice. 2nd ed. [Internet]. Geneva: World Health Organization;c2014. [cited 2020 Oct 15]. Available from: https://www.who.int/publications/i/item/9789241548953.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Papillomavirus Vaccine
- Clinical Benefit of Vaccinating Male Against HPV-related Disease
- Proposal for cervical cancer screening in the era of HPV vaccination
- Current status of cervical cancer and HPV infection in Korea
- Female University Students' HPV-related Knowledge and Influencing Factors on HPV Vaccination