Cancer Res Treat.
2024 Jul;56(3):825-837. 10.4143/crt.2023.753.
The Persistence of Hypertriglyceridemia and the Risk of Early Onset Colorectal Cancer According to Tumor Subsites: A Nationwide Population-Based Study
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Department of Statistics and Actuarial Science, Soongsil University, Seoul, Korea
- 3Department of Medical Statistics, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Department of Internal Medicine, Healthcare Research Institute, Healthcare System Gangnam Center, Seoul National University Hospital, Seoul, Korea
- 5Division of Gastroenterology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2557669
- DOI: http://doi.org/10.4143/crt.2023.753
Abstract
- Purpose
The incidence of early-onset colorectal cancer (EoCRC) is increasing worldwide. The association between hypertriglyceridemia (HTG) and EoCRC risk remains unclear.
Materials and Methods
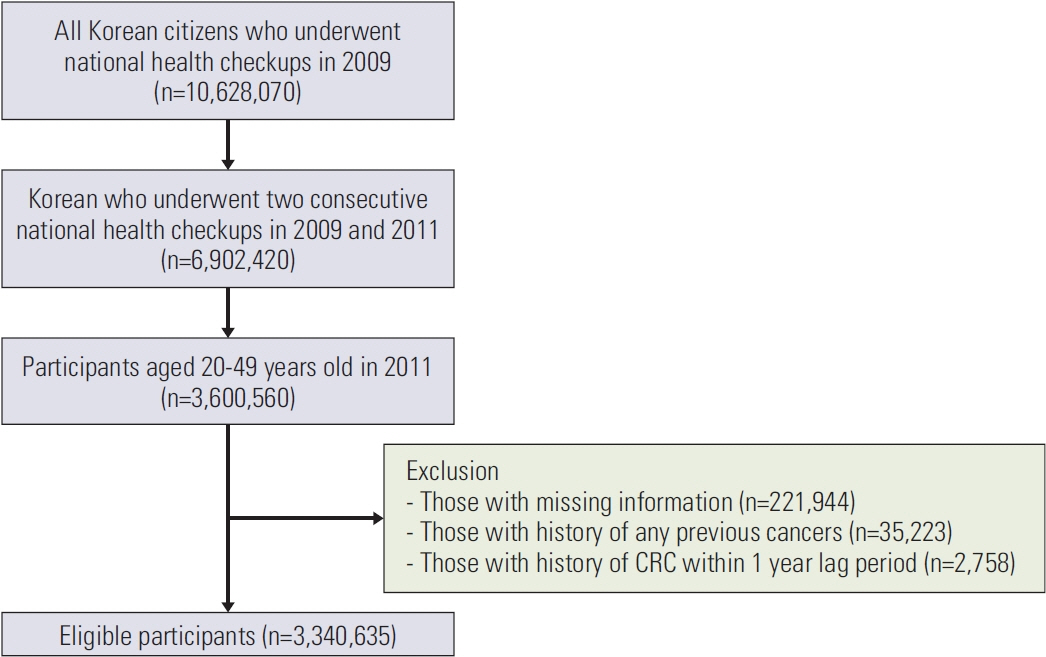
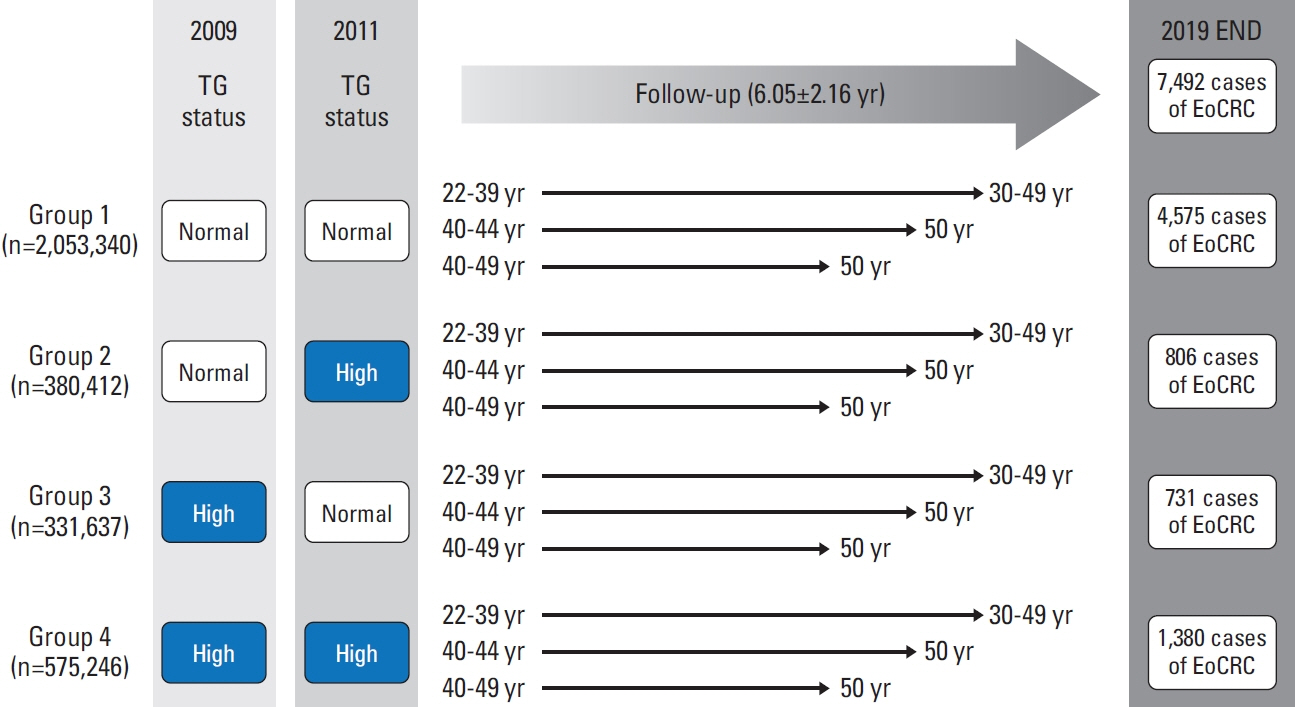
We conducted a nationwide cohort study of 3,340,635 individuals aged 20-49 years who underwent health checkups between 2009 and 2011 under the Korean National Health Insurance Service. HTG was defined as serum triglyceride (TG) level ≥ 150 mg/dL. According to the change in TG status, participants were categorized into persistent normotriglyceridemia (NTG; group 1), NTG to HTG (group 2), HTG to NTG (group 3), and persistent HTG (group 4) groups. The EoCRC incidence was followed up until 2019.
Results
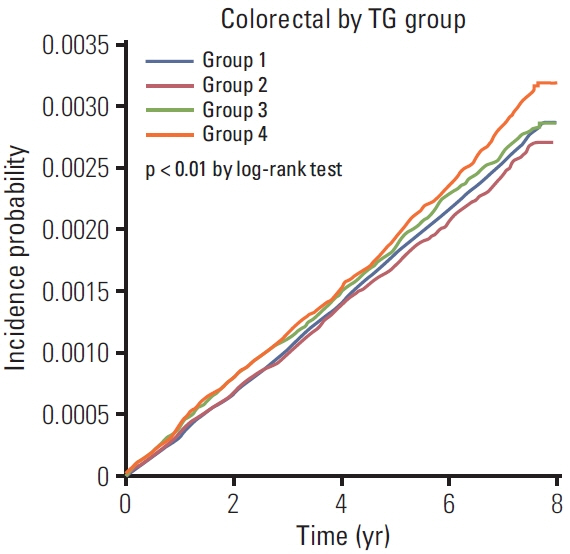
In total, 7,492 EoCRC cases developed after a mean of 6.05 years of follow-up. Group 4 had the highest risk of EoCRC (adjusted hazard ratio [aHR], 1.097; 95% confidence interval [CI], 1.025 to 1.174). While the risk of rectal cancer was significantly increased in groups 3 and 4 (aHR [95% CI], 1.236 [1.076 to 1.419] and 1.175 [1.042-1.325], respectively), no significant risk differences were observed in right colon cancer. In group 4, male sex and diabetes were associated with a further increased risk of EoCRC (aHR [95% CI], 1.149 [1.082 to 1.221] and 1.409 [1.169 to 1.699], respectively). In addition, there was a dose-response relationship between serum TG levels and the risk of EoCRC (p for trends < 0.0001).
Conclusion
Persistent HTG increased the risk of EoCRC, which was significantly higher only for rectal cancer and marginally higher for other colonic subsites.
Figure
Reference
-
References
1. Akimoto N, Ugai T, Zhong R, Hamada T, Fujiyoshi K, Giannakis M, et al. Rising incidence of early-onset colorectal cancer: a call to action. Nat Rev Clin Oncol. 2021; 18:230–43.
Article2. O’Sullivan DE, Sutherland RL, Town S, Chow K, Fan J, Forbes N, et al. Risk factors for early-onset colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022; 20:1229–40.3. Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013; 5:1218–40.
Article4. Das Pradhan A, Glynn RJ, Fruchart JC, MacFadyen JG, Zaharris ES, Everett BM, et al. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N Engl J Med. 2022; 387:1923–34.
Article5. Yang Z, Tang H, Lu S, Sun X, Rao B. Relationship between serum lipid level and colorectal cancer: a systemic review and meta-analysis. BMJ Open. 2022; 12:e052373.
Article6. Borena W, Stocks T, Jonsson H, Strohmaier S, Nagel G, Bjorge T, et al. Serum triglycerides and cancer risk in the metabolic syndrome and cancer (Me-Can) collaborative study. Cancer Causes Control. 2011; 22:291–9.
Article7. Fang Z, He M, Song M. Serum lipid profiles and risk of colorectal cancer: a prospective cohort study in the UK Biobank. Br J Cancer. 2021; 124:663–70.8. Jin EH, Han K, Lee DH, Shin CM, Lim JH, Choi YJ, et al. Association between metabolic syndrome and the risk of colorectal cancer diagnosed before age 50 years according to tumor location. Gastroenterology. 2022; 163:637–48.
Article9. Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011; 123:2292–333.10. Lee CH, Han KD, Kim DH, Kwak MS. The repeatedly elevated fatty liver index is associated with increased mortality: a population-based cohort study. Front Endocrinol (Lausanne). 2021; 12:638615.
Article11. Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016; 22 Suppl 3:1–203.
Article12. Cho JH, Shin CM, Han KD, Yoon H, Park YS, Kim N, et al. Abdominal obesity increases risk for esophageal cancer: a nationwide population-based cohort study of South Korea. J Gastroenterol. 2020; 55:307–16.13. Tulinius H, Sigfusson N, Sigvaldason H, Bjarnadottir K, Tryggvadottir L. Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev. 1997; 6:863–73.14. Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999; 91:1147–54.
Article15. Saydah SH, Platz EA, Rifai N, Pollak MN, Brancati FL, Helzlsouer KJ. Association of markers of insulin and glucose control with subsequent colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2003; 12:412–8.16. Tsushima M, Nomura AM, Lee J, Stemmermann GN. Prospective study of the association of serum triglyceride and glucose with colorectal cancer. Dig Dis Sci. 2005; 50:499–505.
Article17. Ahmed RL, Schmitz KH, Anderson KE, Rosamond WD, Folsom AR. The metabolic syndrome and risk of incident colorectal cancer. Cancer. 2006; 107:28–36.18. Inoue M, Noda M, Kurahashi N, Iwasaki M, Sasazuki S, Iso H, et al. Impact of metabolic factors on subsequent cancer risk: results from a large-scale population-based cohort study in Japan. Eur J Cancer Prev. 2009; 18:240–7.
Article19. van Duijnhoven FJ, Bueno-De-Mesquita HB, Calligaro M, Jenab M, Pischon T, Jansen EH, et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut. 2011; 60:1094–102.20. Agnoli C, Grioni S, Sieri S, Sacerdote C, Vineis P, Tumino R, et al. Colorectal cancer risk and dyslipidemia: a case-cohort study nested in an Italian multicentre cohort. Cancer Epidemiol. 2014; 38:144–51.
Article21. Lu Y, Ness-Jensen E, Hveem K, Martling A. Metabolic predispositions and increased risk of colorectal adenocarcinoma by anatomical location: a large population-based cohort study in Norway. Am J Epidemiol. 2015; 182:883–93.
Article22. Chandler PD, Song Y, Lin J, Zhang S, Sesso HD, Mora S, et al. Lipid biomarkers and long-term risk of cancer in the Women’s Health Study. Am J Clin Nutr. 2016; 103:1397–407.23. Katzke VA, Sookthai D, Johnson T, Kuhn T, Kaaks R. Blood lipids and lipoproteins in relation to incidence and mortality risks for CVD and cancer in the prospective EPIC-Heidelberg cohort. BMC Med. 2017; 15:218.
Article24. Li X, Chen H, Wang G, Feng X, Lyu Z, Wei L, et al. Metabolic syndrome components and the risk of colorectal cancer: a population-based prospective study in Chinese men. Front Oncol. 2019; 9:1047.
Article25. Hsu SH, Syu DK, Chen YC, Liu CK, Sun CA, Chen M. The association between hypertriglyceridemia and colorectal cancer: a long-term community cohort study in Taiwan. Int J Environ Res Public Health. 2022; 19:7804.
Article26. Jung YS, Ryu S, Chang Y, Yun KE, Park JH, Kim HJ, et al. Risk factors for colorectal neoplasia in persons aged 30 to 39 years and 40 to 49 years. Gastrointest Endosc. 2015; 81:637–45.
Article27. Chen J, Iverson D. Estrogen in obesity-associated colon cancer: friend or foe? Protecting postmenopausal women but promoting late-stage colon cancer. Cancer Causes Control. 2012; 23:1767–73.
Article28. Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005; 97:1679–87.
Article29. Coleman O, Ecker M, Haller D. Dysregulated lipid metabolism in colorectal cancer. Curr Opin Gastroenterol. 2022; 38:162–7.
Article30. Ecker J, Benedetti E, Kindt AS, Horing M, Perl M, Machmuller AC, et al. The colorectal cancer lipidome: identification of a robust tumor-specific lipid species signature. Gastroenterology. 2021; 161:910–23.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Proposal of age definition for early-onset gastric cancer based on the Korean Gastric Cancer Association nationwide survey data: a retrospective observational study
- Impact of Diet on Colorectal Cancer Progression and Prevention: From Nutrients to Neoplasms
- Association between the Persistence of Obesity and the Risk of Gastric Cancer: A Nationwide Population-Based Study
- Data on the Characteristics and the Survival of Korean Patients With Colorectal Cancer From the Korea Central Cancer Registry
- Early-onset Colorectal Cancer