Cancer Res Treat.
2024 Jul;56(3):802-808. 10.4143/crt.2023.1186.
Implementation of BRCA Test among Young Breast Cancer Patients in South Korea: A Nationwide Cohort Study
- Affiliations
-
- 1Division of Breast Surgery, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2557667
- DOI: http://doi.org/10.4143/crt.2023.1186
Abstract
- Purpose
This study aimed to investigate the frequency of BRCA testing and related factors among young breast cancer patients (age < 40 years) in South Korea.
Materials and Methods
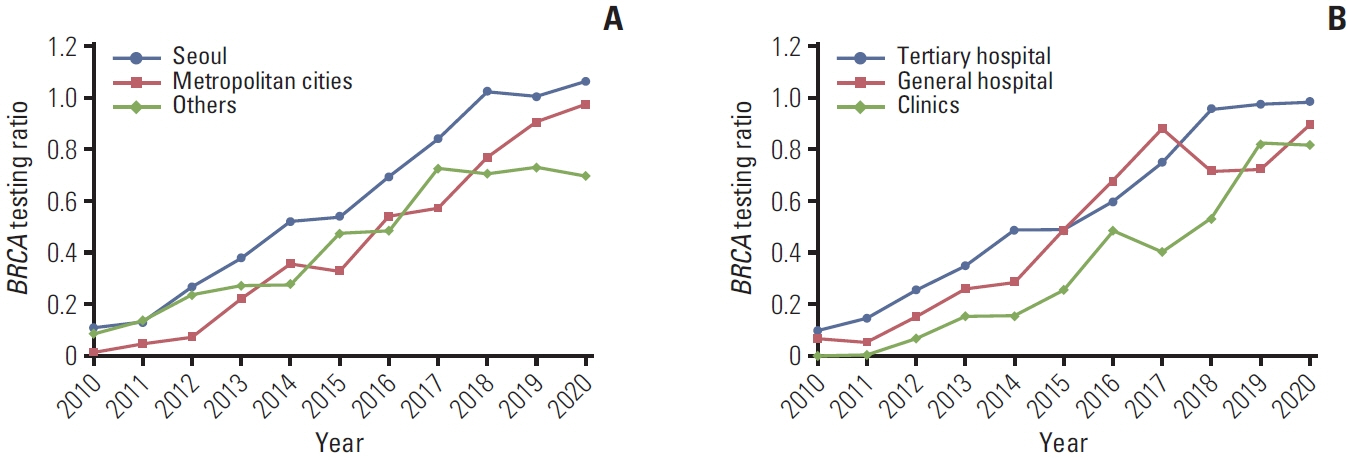
We conducted a nationwide retrospective cohort study using data from the Health Insurance Review and Assessment claims. Newly diagnosed breast cancer patients younger than 40 were included. Annual BRCA testing ratios (number of BRCA test recipients/the number of patients undergoing breast cancer surgery in each year) were analyzed by region and health care delivery system. We investigated the location of breast cancer diagnosis and BRCA testing.
Results
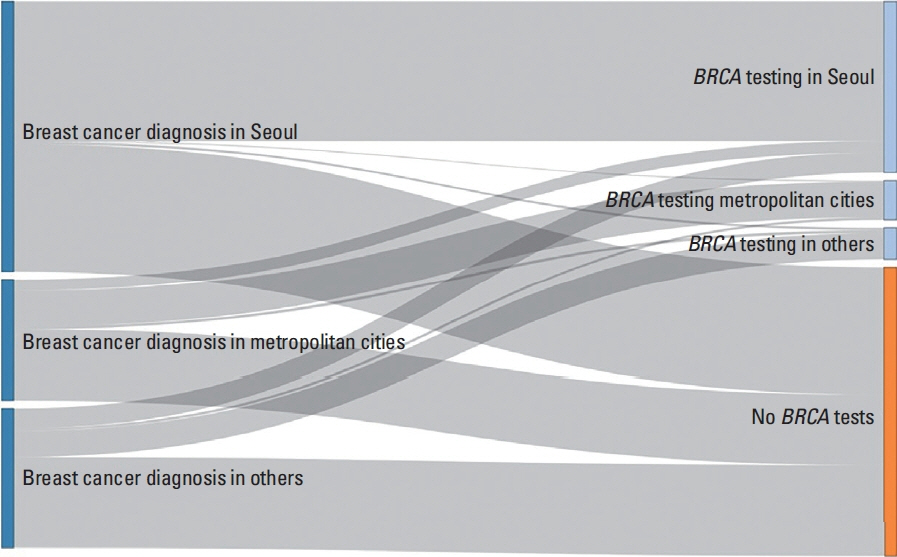
From January 2010 to December 2020, there were 25,665 newly diagnosed young breast cancer patients, of whom 12,186 (47.5%) underwent BRCA testing. The BRCA testing ratios increased gradually from 0.084 (154/1,842) in 2010 to 0.961 (1,975/2,055) in 2020. Medical aid (vs. health insurance) and undergoing surgery in metropolitan cities or others (vs. Seoul), general hospitals, and clinics (vs. tertiary hospitals) were associated with a lower likelihood of BRCA testing. While 97.8% of the patients diagnosed in Seoul underwent BRCA testing in Seoul, 22.9% and 29.2% of patients who were diagnosed in metropolitan areas and other regions moved to Seoul and underwent BRCA testing, respectively.
Conclusion
The frequency of BRCA testing has increased over time in South Korea, with Seoul showing a particularly high rate of testing. About one-quarter of patients diagnosed with breast cancer outside of Seoul moved to Seoul and underwent BRCA testing.
Figure
Reference
-
References
1. Valencia OM, Samuel SE, Viscusi RK, Riall TS, Neumayer LA, Aziz H. The role of genetic testing in patients with breast cancer: a review. JAMA Surg. 2017; 152:589–94.
Article2. U. S. Preventive Services Task Force, Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force recommendation statement. JAMA. 2019; 322:652–65.
Article3. Tomasello G, Gambini D, Petrelli F, Azzollini J, Arcana C, Ghidini M, et al. Characterization of the HER2 status in BRCA-mutated breast cancer: a single institutional series and systematic review with pooled analysis. ESMO Open. 2022; 7:100531.
Article4. Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017; 377:523–33.
Article5. Tutt AN, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021; 384:2394–405.
Article6. Lee EG, Kang HJ, Lim MC, Park B, Park SJ, Jung SY, et al. Different patterns of risk reducing decisions in affected or unaffected BRCA pathogenic variant carriers. Cancer Res Treat. 2019; 51:280–8.
Article7. Tischler J, Crew KD, Chung WK. Cases in precision medicine: the role of tumor and germline genetic testing in breast cancer management. Ann Intern Med. 2019; 171:925–30.
Article8. Stoll K, Kubendran S, Cohen SA. The past, present and future of service delivery in genetic counseling: keeping up in the era of precision medicine. Am J Med Genet C Semin Med Genet. 2018; 178:24–37.
Article9. Radford C, Prince A, Lewis K, Pal T. Factors which impact the delivery of genetic risk assessment services focused on inherited cancer genomics: expanding the role and reach of certified genetics professionals. J Genet Couns. 2014; 23:522–30.
Article10. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017; 32:718–28.
Article11. Jeong JH, Jung J, Kim HJ, Lee JW, Ko BS, Son BH, et al. Domestic medical travel from non-Seoul regions to Seoul for initial breast cancer treatment: a nationwide cohort study. Ann Surg Treat Res. 2023; 104:71–9.
Article12. Chung IY, Lee J, Park S, Lee JW, Youn HJ, Hong JH, et al. Nationwide analysis of treatment patterns for Korean breast cancer survivors using National Health Insurance Service Data. J Korean Med Sci. 2018; 33:e276.
Article13. Stenehjem DD, Telford C, Unni SK, Bauer H, Sainski A, Deka R, et al. BRCA testing and outcomes in women with breast cancer. Breast Cancer Res Treat. 2021; 186:839–50.14. Martin AP, Downing J, Collins B, Godman B, Alfirevic A, Greenhalgh KL, et al. Examining the uptake of predictive BRCA testing in the UK: findings and implications. Eur J Hum Genet. 2021; 29:699–708.
Article15. Bellcross CA, Peipins LA, McCarty FA, Rodriguez JL, Hawkins NA, Hensley Alford S, et al. Characteristics associated with genetic counseling referral and BRCA1/2 testing among women in a large integrated health system. Genet Med. 2015; 17:43–50.
Article16. Lee J, Kim S, Kang E, Park S, Kim Z, Lee MH, et al. Influence of the Angelina Jolie announcement and insurance reimbursement on practice patterns for hereditary breast cancer. J Breast Cancer. 2017; 20:203–7.
Article17. Guo F, Scholl M, Fuchs EL, Berenson AB, Kuo YF. BRCA testing in unaffected young women in the United States, 2006-2017. Cancer. 2020; 126:337–43.
Article18. Kolor K, Chen Z, Grosse SD, Rodriguez JL, Green RF, Dotson WD, et al. BRCA genetic testing and receipt of preventive interventions among women aged 18-64 years with employer-sponsored health insurance in nonmetropolitan and metropolitan areas - United States, 2009-2014. MMWR Surveill Summ. 2017; 66:1–11.19. Kim N, Kong SY, Yoo J, Kim DH, Seo SH, Kim J. Current issues, challenges, and future perspectives of genetic counseling in Korea. Ann Lab Med. 2022; 42:314–20.
Article20. Bahk J, Kang HY, Khang YH. Trends in life expectancy among medical aid beneficiaries and National Health Insurance beneficiaries in Korea between 2004 and 2017. BMC Public Health. 2019; 19:1137.
Article21. Schlich-Bakker KJ, ten Kroode HF, Warlam-Rodenhuis CC, van den Bout J, Ausems MG. Barriers to participating in genetic counseling and BRCA testing during primary treatment for breast cancer. Genet Med. 2007; 9:766–77.
Article22. Geer KP, Ropka ME, Cohn WF, Jones SM, Miesfeldt S. Factors influencing patients’ decisions to decline cancer genetic counseling services. J Genet Couns. 2001; 10:25–40.
Article23. Foster C, Evans DG, Eeles R, Eccles D, Ashley S, Brooks L, et al. Non-uptake of predictive genetic testing for BRCA1/2 among relatives of known carriers: attributes, cancer worry, and barriers to testing in a multicenter clinical cohort. Genet Test. 2004; 8:23–9.24. Kim H, Cho DY, Choi DH, Choi SY, Shin I, Park W, et al. Characteristics and spectrum of BRCA1 and BRCA2 mutations in 3,922 Korean patients with breast and ovarian cancer. Breast Cancer Res Treat. 2012; 134:1315–26.
Article25. Son BH, Ahn SH, Kim SW, Kang E, Park SK, Lee MH, et al. Prevalence of BRCA1 and BRCA2 mutations in non-familial breast cancer patients with high risks in Korea: the Korean Hereditary Breast Cancer (KOHBRA) Study. Breast Cancer Res Treat. 2012; 133:1143–52.
Article26. Ministry of Health and Welfare. Bogunbokjibu Gosi [Ministry of Health and Welfare notice] (Vol. 2020-135) [Internet]. Sejong: Ministry of Health and Welfare; c2020 [cited 2023 Sep 12]. Available from: https://www.mohw.go.kr/attachPreview.es?bid=0026&list_no=355193&seq=1.27. Ministry of Food and Drug Safety. 2019 Drug approval report [Internet]. Cheongju: Ministry of Food and Drug Safety; c2020 [cited 2023 Sep 17]. Available from: https://www.mfds.go.kr/eng/brd/m_19/view.do?seq=70435.28. Jeong MJ. Lynpaza approved as primary treatment for breast and prostate cancer [Internet]. Seoul: Korea Biomedical Review; c2023 [cited 2023 Sep 17]. Available from: https://www.koreabiomed.com/news/articleView.html?idxno=20539.29. Park JS, Shin S, Lee YJ, Lee ST, Nam EJ, Han JW, et al. Implication and influence of multigene panel testing with genetic counseling in Korean patients with BRCA1/2 mutation-negative breast cancer. Cancer Res Treat. 2022; 54:1099–110.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Korean Hereditary Breast Cancer
- The Korean Hereditary Breast Cancer Study: Review and Future Perspectives
- Clinicopathological characteristics of BRCA-associated breast cancer in Asian patients
- Associations between BRCA Mutations in High-Risk Breast Cancer Patients and Familial Cancers Other than Breast or Ovary
- Short-term Impact of Hormone Replacement Therapy on Risk of Breast Cancer in BRCA Mutation Carriers: A Nationwide Study in South Korea