J Pathol Transl Med.
2020 Jul;54(4):265-275. 10.4132/jptm.2020.04.07.
Clinicopathological characteristics of BRCA-associated breast cancer in Asian patients
- Affiliations
-
- 1Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 2Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 3Department of Surgery, Daerim St. Mary’s Hospital, Seoul, Korea
- KMID: 2504557
- DOI: http://doi.org/10.4132/jptm.2020.04.07
Abstract
BRCA 1/2 germline mutations account for the majority of hereditary breast cancers. Since the identification of theBRCA genes, several attempts have been made to define the clinicopathological characteristics ofBRCA -associated breast cancer in comparison with sporadic breast cancer. Asians constitute 60% of the world population, and although the incidence of breast cancer in Asia remains low compared to the West, breast cancer is the most prevalent female cancer in the region. The epidemiological aspects of breast cancer are different between Asians and Caucasians. Asian patients present with breast cancer at a younger age than Western patients. The contributions ofBRCA1/2 mutations to breast cancer incidence are expected to differ between Asians and Caucasians, and the different genetic backgrounds among races are likely to influence the breast cancer phenotypes. However, most large-scale studies on the clinicopathological characteristics ofBRCA -associated breast cancer have been on Western patients, while studies on Asian populations were small and sporadic. In this review, we provide an overview of the clinical and pathological characteristics ofBRCA -associated breast cancer, incorporating findings on Asian patients.
Figure
Cited by 1 articles
-
Harnessing Institutionally Developed Clinical Targeted Sequencing to Improve Patient Survival in Breast Cancer: A Seven-Year Experience
Jiwon Koh, Jinyong Kim, Go-Un Woo, Hanbaek Yi, So Yean Kwon, Jeongmin Seo, Jeong Mo Bae, Jung Ho Kim, Jae Kyung Won, Han Suk Ryu, Yoon Kyung Jeon, Dae-Won Lee, Miso Kim, Tae-Yong Kim, Kyung-Hun Lee, Tae-You Kim, Jee-Soo Lee, Moon-Woo Seong, Sheehyun Kim, Sungyoung Lee, Hongseok Yun, Myung Geun Song, Jaeyong Choi, Jong-Il Kim, Seock-Ah Im
Cancer Res Treat. 2025;57(2):443-456. doi: 10.4143/crt.2024.296.
Reference
-
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
Article2. Ripperger T, Gadzicki D, Meindl A, Schlegelberger B. Breast cancer susceptibility: current knowledge and implications for genetic counselling. Eur J Hum Genet. 2009; 17:722–31.
Article3. Fackenthal JD, Olopade OI. Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat Rev Cancer. 2007; 7:937–48.4. Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998; 62:676–89.5. Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003; 72:1117–30.6. Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007; 25:1329–33.7. Ferla R, Calo V, Cascio S, et al. Founder mutations in BRCA1 and BRCA2 genes. Ann Oncol. 2007; 18 Suppl 6:vi93–8.8. Daly MB, Axilbund JE, Buys S, et al. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw. 2010; 8:562–94.
Article9. Gonzalez-Angulo AM, Timms KM, Liu S, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res. 2011; 17:1082–9.10. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
Article11. Shin HR, Carlos MC, Varghese C. Cancer control in the Asia Pacific region: current status and concerns. Jpn J Clin Oncol. 2012; 42:867–81.
Article12. Ko BS, Noh WC, Kang SS, et al. Changing patterns in the clinical characteristics of Korean breast cancer from 1996-2010 using an online nationwide breast cancer database. J Breast Cancer. 2012; 15:393–400.
Article13. Kim H, Choi DH. Distribution of BRCA1 and BRCA2 mutations in Asian patients with breast cancer. J Breast Cancer. 2013; 16:357–65.14. Yang L, Parkin DM, Ferlay J, Li L, Chen Y. Estimates of cancer incidence in China for 2000 and projections for 2005. Cancer Epidemiol Biomarkers Prev. 2005; 14:243–50.15. Kurian AW, Fish K, Shema SJ, Clarke CA. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010; 12:R99.
Article16. Anglian Breast Cancer Study Group. Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Anglian Breast Cancer Study Group. Br J Cancer. 2000; 83:1301–8.17. Lang GT, Shi JX, Hu X, et al. The spectrum of BRCA mutations and characteristics of BRCA-associated breast cancers in China: screening of 2,991 patients and 1,043 controls by next-generation sequencing. Int J Cancer. 2017; 141:129–42.18. Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994; 343:692–5.19. Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995; 56:265–71.20. Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997; 336:1401–8.21. Han SA, Park SK, Ahn SH, et al. The breast and ovarian cancer risks in Korea due to inherited mutations in BRCA1 and BRCA2: a preliminary report. J Breast Cancer. 2009; 12:92–9.22. Ikeda N, Miyoshi Y, Ikeda N, Yoneda K, Kinoshita M, Noguchi S. Frequency of BRCA1 and BRCA2 germline mutations detected by protein truncation test and cumulative risks of breast and ovarian cancer among mutation carriers in Japanese breast cancer families. J Korean Breast Cancer Soc. 2002; 5:194–201.23. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017; 377:523–33.24. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018; 379:753–63.25. Han SA, Kim SW, Kang E, et al. The prevalence of BRCA mutations among familial breast cancer patients in Korea: results of the Korean Hereditary Breast Cancer study. Fam Cancer. 2013; 12:75–81.26. Sugano K, Nakamura S, Ando J, et al. Cross-sectional analysis of germline BRCA1 and BRCA2 mutations in Japanese patients suspected to have hereditary breast/ovarian cancer. Cancer Sci. 2008; 99:1967–76.27. Kwong A, Ng EK, Wong CL, et al. Identification of BRCA1/2 founder mutations in Southern Chinese breast cancer patients using gene sequencing and high resolution DNA melting analysis. PLoS One. 2012; 7:e43994.28. Valarmathi MT, Sawhney M, Deo SS, Shukla NK, Das SN. Novel germline mutations in the BRCA1 and BRCA2 genes in Indian breast and breast-ovarian cancer families. Hum Mutat. 2004; 23:205.29. Kwong A, Wong LP, Wong HN, et al. Clinical and pathological characteristics of Chinese patients with BRCA related breast cancer. Hugo J. 2009; 3:63–76.
Article30. Yip CH, Taib NA, Choo WY, Rampal S, Thong MK, Teo SH. Clinical and pathologic differences between BRCA1-, BRCA2-, and non-BRCA-associated breast cancers in a multiracial developing country. World J Surg. 2009; 33:2077–81.31. Wooster R, Neuhausen SL, Mangion J, et al. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q1213. Science. 1994; 265:2088–90.32. Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999; 91:1310–6.33. Thompson D, Easton DF; Breast Cancer Linkage Consortium. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002; 94:1358–65.34. FitzGerald MG, MacDonald DJ, Krainer M, et al. Germ-line BRCA1 mutations in Jewish and non-Jewish women with earlyonset breast cancer. N Engl J Med. 1996; 334:143–9.35. Shattuck-Eidens D, Oliphant A, McClure M, et al. BRCA1 sequence analysis in women at high risk for susceptibility mutations: risk factor analysis and implications for genetic testing. JAMA. 1997; 278:1242–50.36. Malone KE, Daling JR, Thompson JD, O’Brien CA, Francisco LV, Ostrander EA. BRCA1 mutations and breast cancer in the general population: analyses in women before age 35 years and in women before age 45 years with first-degree family history. JAMA. 1998; 279:922–9.37. Hodgson SV, Heap E, Cameron J, et al. Risk factors for detecting germline BRCA1 and BRCA2 founder mutations in Ashkenazi Jewish women with breast or ovarian cancer. J Med Genet. 1999; 36:369–73.38. Warner E, Foulkes W, Goodwin P, et al. Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst. 1999; 91:1241–7.39. Langston AA, Malone KE, Thompson JD, Daling JR, Ostrander EA. BRCA1 mutations in a population-based sample of young women with breast cancer. N Engl J Med. 1996; 334:137–42.40. Peto J, Collins N, Barfoot R, et al. Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst. 1999; 91:943–9.41. Ligtenberg MJ, Hogervorst FB, Willems HW, et al. Characteristics of small breast and/or ovarian cancer families with germline mutations in BRCA1 and BRCA2. Br J Cancer. 1999; 79:1475–8.42. Krainer M, Silva-Arrieta S, FitzGerald MG, et al. Differential contributions of BRCA1 and BRCA2 to early-onset breast cancer. N Engl J Med. 1997; 336:1416–21.43. Hartge P, Struewing JP, Wacholder S, Brody LC, Tucker MA. The prevalence of common BRCA1 and BRCA2 mutations among Ashkenazi Jews. Am J Hum Genet. 1999; 64:963–70.44. Hopper JL, Jenkins MA. Modeling the probability that Ashkenazi Jewish women carry a founder mutation in BRCA1 or BRCA2. Am J Hum Genet. 1999; 65:1771–6.45. Foulkes WD, Brunet JS, Warner E, et al. The importance of a family history of breast cancer in predicting the presence of a BRCA mutation. Am J Hum Genet. 1999; 65:1776–9.46. Li WF, Hu Z, Rao NY, et al. The prevalence of BRCA1 and BRCA2 germline mutations in high-risk breast cancer patients of Chinese Han nationality: two recurrent mutations were identified. Breast Cancer Res Treat. 2008; 110:99–109.47. Zhang J, Pei R, Pang Z, et al. Prevalence and characterization of BRCA1 and BRCA2 germline mutations in Chinese women with familial breast cancer. Breast Cancer Res Treat. 2012; 132:421–8.48. De Leon Matsuda ML, Liede A, Kwan E, et al. BRCA1 and BRCA2 mutations among breast cancer patients from the Philippines. Int J Cancer. 2002; 98:596–603.49. Marcus JN, Watson P, Page DL, et al. Hereditary breast cancer: pathobiology, prognosis, and BRCA1 and BRCA2 gene linkage. Cancer. 1996; 77:697–709.50. Johannsson OT, Ranstam J, Borg A, Olsson H. Survival of BRCA1 breast and ovarian cancer patients: a population-based study from southern Sweden. J Clin Oncol. 1998; 16:397–404.51. Robson M, Gilewski T, Haas B, et al. BRCA-associated breast cancer in young women. J Clin Oncol. 1998; 16:1642–9.52. Noguchi S, Kasugai T, Miki Y, Fukutomi T, Emi M, Nomizu T. Clinicopathologic analysis of BRCA1- or BRCA2-associated hereditary breast carcinoma in Japanese women. Cancer. 1999; 85:2200–5.53. Couch FJ, DeShano ML, Blackwood MA, et al. BRCA1 mutations in women attending clinics that evaluate the risk of breast cancer. N Engl J Med. 1997; 336:1409–15.54. Verhoog LC, Brekelmans CT, Seynaeve C, et al. Survival and tumour characteristics of breast-cancer patients with germline mutations of BRCA1. Lancet. 1998; 351:316–21.55. Johannsson OT, Idvall I, Anderson C, et al. Tumour biological features of BRCA1-induced breast and ovarian cancer. Eur J Cancer. 1997; 33:362–71.56. Armes JE, Egan AJ, Southey MC, et al. The histologic phenotypes of breast carcinoma occurring before age 40 years in women with and without BRCA1 or BRCA2 germline mutations: a population-based study. Cancer. 1998; 83:2335–45.57. Garcia-Patiño E, Gomendio B, Provencio M, et al. Germ-line BRCA1 mutations in women with sporadic breast cancer: clinical correlations. J Clin Oncol. 1998; 16:115–20.58. Yu JH, Lee JW, Son BH, et al. Characteristics of BRCA1/2 mutation-positive breast cancers in Korea: a comparison study based on multicenter data and the Korean Breast Cancer Registry. J Breast Cancer. 2014; 17:129–35.59. Foulkes WD, Wong N, Brunet JS, et al. Germ-line BRCA1 mutation is an adverse prognostic factor in Ashkenazi Jewish women with breast cancer. Clin Cancer Res. 1997; 3(12 Pt 1):2465–9.60. Verhoog LC, Brekelmans CT, Seynaeve C, et al. Survival in hereditary breast cancer associated with germline mutations of BRCA2. J Clin Oncol. 1999; 17:3396–402.61. Wagner TM, Moslinger RA, Muhr D, et al. BRCA1-related breast cancer in Austrian breast and ovarian cancer families: specific BRCA1 mutations and pathological characteristics. Int J Cancer. 1998; 77:354–60.62. Eisinger F, Noguès C, Birnbaum D, Jacquemier J, Sobol H. Low frequency of lymph-node metastasis in BRCA1-associated breast cancer. Lancet. 1998; 351:1633–4.63. Stoppa-Lyonnet D, Ansquer Y, Dreyfus H, et al. Familial invasive breast cancers: worse outcome related to BRCA1 mutations. J Clin Oncol. 2000; 18:4053–9.64. Robson ME, Chappuis PO, Satagopan J, et al. A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res. 2004; 6:R8–17.
Article65. Ansquer Y, Gautier C, Fourquet A, Asselain B, Stoppa-Lyonnet D. Survival in early-onset BRCA1 breast-cancer patients. Institut Curie Breast Cancer Group. Lancet. 1998; 352:541.66. Moran MS, Yang Q, Harris LN, Jones B, Tuck DP, Haffty BG. Long-term outcomes and clinicopathologic differences of African-American versus white patients treated with breast conservation therapy for early-stage breast cancer. Cancer. 2008; 113:2565–74.
Article67. Foulkes WD, Chappuis PO, Wong N, et al. Primary node negative breast cancer in BRCA1 mutation carriers has a poor outcome. Ann Oncol. 2000; 11:307–13.68. Nilsson MP, Hartman L, Idvall I, Kristoffersson U, Johannsson OT, Loman N. Long-term prognosis of early-onset breast cancer in a population-based cohort with a known BRCA1/2 mutation status. Breast Cancer Res Treat. 2014; 144:133–42.69. Nicoletto MO, Donach M, De Nicolo A, Artioli G, Banna G, Monfardini S. BRCA-1 and BRCA-2 mutations as prognostic factors in clinical practice and genetic counselling. Cancer Treat Rev. 2001; 27:295–304.
Article70. El-Tamer M, Russo D, Troxel A, et al. Survival and recurrence after breast cancer in BRCA1/2 mutation carriers. Ann Surg Oncol. 2004; 11:157–64.71. Albano WA, Recabaren JA, Lynch HT, et al. Natural history of hereditary cancer of the breast and colon. Cancer. 1982; 50:360–3.
Article72. Porter DE, Cohen BB, Wallace MR, et al. Breast cancer incidence, penetrance and survival in probable carriers of BRCA1 gene mutation in families linked to BRCA1 on chromosome 17q12-21. Br J Surg. 1994; 81:1512–5.73. Rennert G, Bisland-Naggan S, Barnett-Griness O, et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007; 357:115–23.74. Bordeleau L, Panchal S, Goodwin P. Prognosis of BRCA-associated breast cancer: a summary of evidence. Breast Cancer Res Treat. 2010; 119:13–24.75. Lee EH, Park SK, Park B, et al. Effect of BRCA1/2 mutation on short-term and long-term breast cancer survival: a systematic review and meta-analysis. Breast Cancer Res Treat. 2010; 122:11–25.76. Xu J, Wang B, Zhang Y, Li R, Wang Y, Zhang S. Clinical implications for BRCA gene mutation in breast cancer. Mol Biol Rep. 2012; 39:3097–102.77. Lucassen A, Watson E, Eccles D. Evidence based case report: advice about mammography for a young woman with a family history of breast cancer. BMJ. 2001; 322:1040–2.
Article78. Chappuis PO, Nethercot V, Foulkes WD. Clinico-pathological characteristics of BRCA1- and BRCA2-related breast cancer. Semin Surg Oncol. 2000; 18:287–95.79. Seynaeve C, Verhoog LC, van de Bosch LM, et al. Ipsilateral breast tumour recurrence in hereditary breast cancer following breast-conserving therapy. Eur J Cancer. 2004; 40:1150–8.
Article80. Haffty BG, Harrold E, Khan AJ, et al. Outcome of conservatively managed early-onset breast cancer by BRCA1/2 status. Lancet. 2002; 359:1471–7.81. Pathology of familial breast cancer: differences between breast cancers in carriers of BRCA1 or BRCA2 mutations and sporadic cases. Breast Cancer Linkage Consortium. Lancet. 1997; 349:1505–10.82. Eisinger F, Jacquemier J, Charpin C, et al. Mutations at BRCA1: the medullary breast carcinoma revisited. Cancer Res. 1998; 58:1588–92.83. Lakhani SR, Jacquemier J, Sloane JP, et al. Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst. 1998; 90:1138–45.84. Honrado E, Benítez J, Palacios J. The molecular pathology of hereditary breast cancer: genetic testing and therapeutic implications. Mod Pathol. 2005; 18:1305–20.
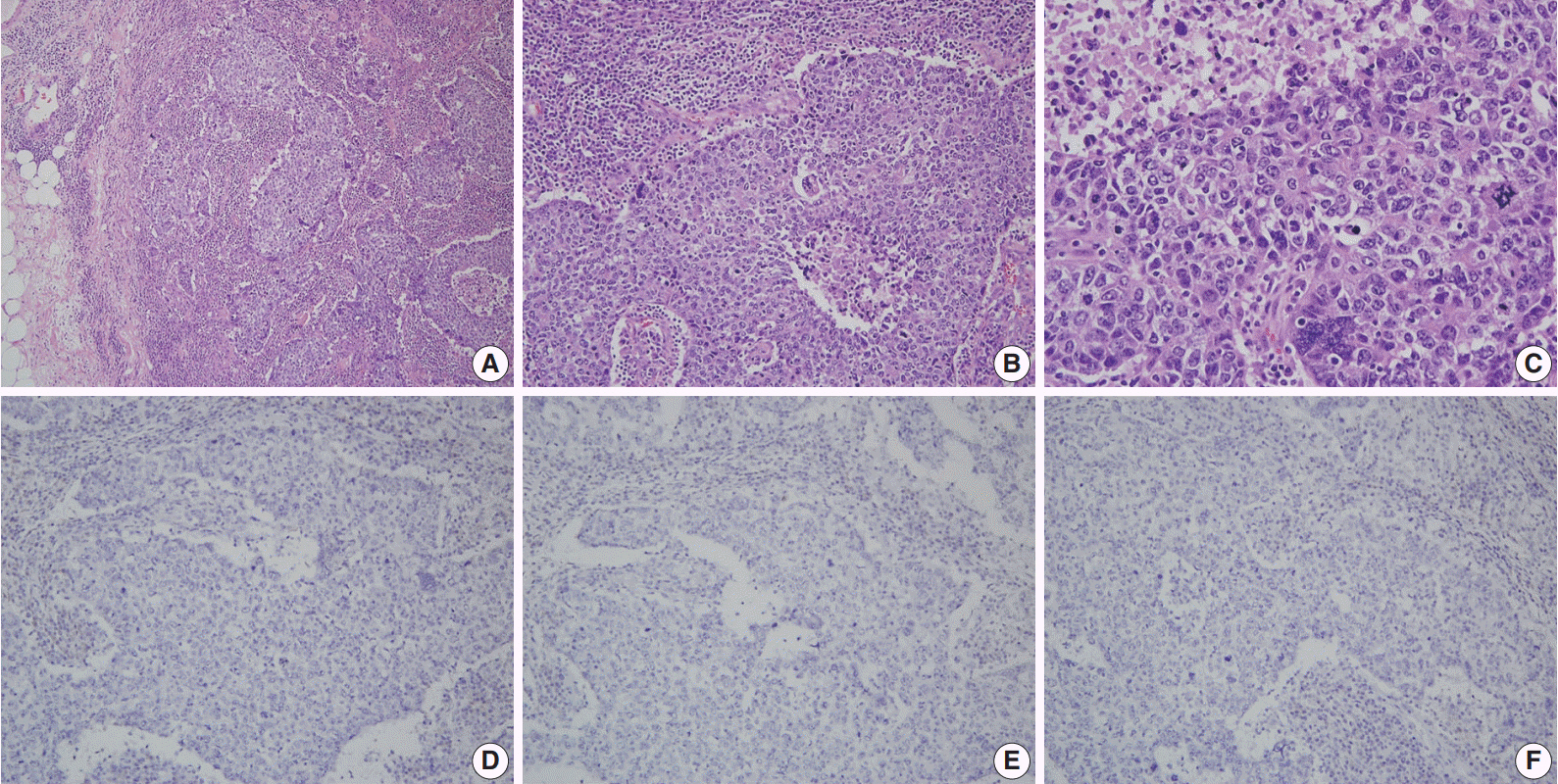
Article85. Lynch BJ, Holden JA, Buys SS, Neuhausen SL, Gaffney DK. Pathobiologic characteristics of hereditary breast cancer. Hum Pathol. 1998; 29:1140–4.
Article86. Eerola H, Heikkilä P, Tamminen A, Aittomäki K, Blomqvist C, Nevanlinna H. Histopathological features of breast tumours in BRCA1, BRCA2 and mutation-negative breast cancer families. Breast Cancer Res. 2005; 7:R93–100.
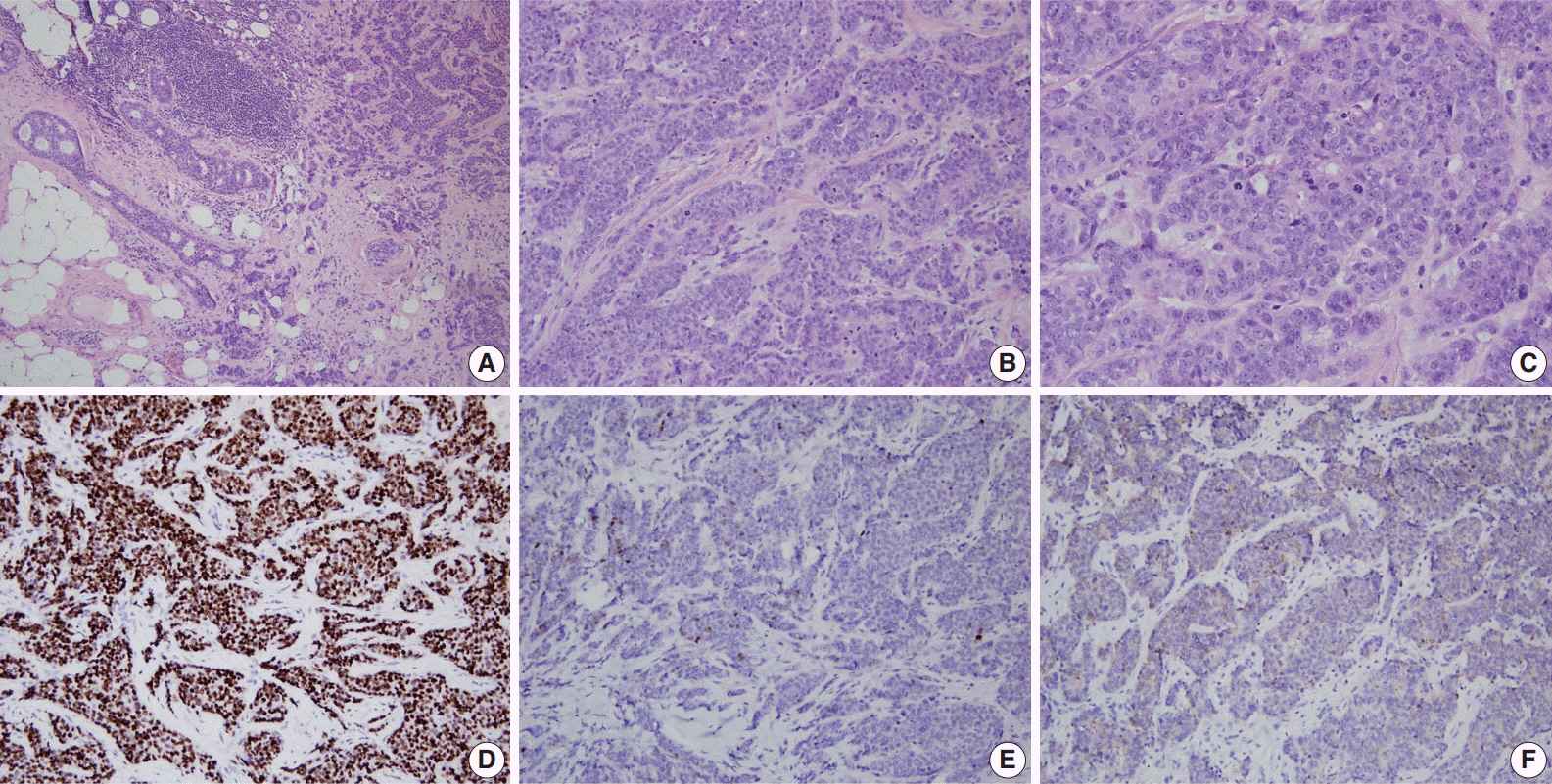
Article87. Agnarsson BA, Jonasson JG, Björnsdottir IB, Barkardottir RB, Egilsson V, Sigurdsson H. Inherited BRCA2 mutation associated with high grade breast cancer. Breast Cancer Res Treat. 1998; 47:121–7.88. Lakhani SR, Van De Vijver MJ, Jacquemier J, et al. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol. 2002; 20:2310–8.89. Palacios J, Honrado E, Osorio A, et al. Immunohistochemical characteristics defined by tissue microarray of hereditary breast cancer not attributable to BRCA1 or BRCA2 mutations: differences from breast carcinomas arising in BRCA1 and BRCA2 mutation carriers. Clin Cancer Res. 2003; 9(10 Pt 1):3606–14.90. Foulkes WD, Metcalfe K, Sun P, et al. Estrogen receptor status in BRCA1- and BRCA2-related breast cancer: the influence of age, grade, and histological type. Clin Cancer Res. 2004; 10:2029–34.91. Osin PP, Lakhani SR. The pathology of familial breast cancer: immunohistochemistry and molecular analysis. Breast Cancer Res. 1999; 1:36–40.
Article92. Eisinger F, Jacquemier J, Charafe-Jauffret E, Rio MC, Birnbaum D, Sobol H. More about: multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst. 1999; 91:1421–2.93. Armes JE, Trute L, White D, et al. Distinct molecular pathogeneses of early-onset breast cancers in BRCA1 and BRCA2 mutation carriers: a population-based study. Cancer Res. 1999; 59:2011–7.94. Grushko TA, Blackwood MA, Schumm PL, et al. Molecular-cytogenetic analysis of HER-2/neu gene in BRCA1-associated breast cancers. Cancer Res. 2002; 62:1481–8.95. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010; 363:1938–48.
Article96. Mavaddat N, Barrowdale D, Andrulis IL, et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012; 21:134–47.97. Atchley DP, Albarracin CT, Lopez A, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008; 26:4282–8.
Article98. Sharma P, Klemp JR, Kimler BF, et al. Germline BRCA mutation evaluation in a prospective triple-negative breast cancer registry: implications for hereditary breast and/or ovarian cancer syndrome testing. Breast Cancer Res Treat. 2014; 145:707–14.99. Noh JM, Han BK, Choi DH, et al. Association between BRCA mutation status, pathological findings, and magnetic resonance imaging features in patients with breast cancer at risk for the mutation. J Breast Cancer. 2013; 16:308–14.100. Claus EB, Petruzella S, Matloff E, Carter D. Prevalence of BRCA1 and BRCA2 mutations in women diagnosed with ductal carcinoma in situ. JAMA. 2005; 293:964–9.101. Hoogerbrugge N, Bult P, de Widt-Levert LM, et al. High prevalence of premalignant lesions in prophylactically removed breasts from women at hereditary risk for breast cancer. J Clin Oncol. 2003; 21:41–5.
Article102. Kauff ND, Brogi E, Scheuer L, et al. Epithelial lesions in prophylactic mastectomy specimens from women with BRCA mutations. Cancer. 2003; 97:1601–8.103. Adem C, Reynolds C, Soderberg CL, et al. Pathologic characteristics of breast parenchyma in patients with hereditary breast carcinoma, including BRCA1 and BRCA2 mutation carriers. Cancer. 2003; 97:1–11.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hereditary Breast Cancer in Korea: A Review of the Literature
- The Incidence of Occult Malignancy in Contralateral Risk Reducing Mastectomy Among Affected Breast Cancer Gene Mutation Carriers in South Korea
- Occurrence of contralateral breast cancer in a BRCA-positive breast cancer patient who underwent free TRAM flap reconstruction: a case report
- Implementation of BRCA Test among Young Breast Cancer Patients in South Korea: A Nationwide Cohort Study
- Magnetic Resonance Imaging of Breast Cancer Patients with BRCA Mutation