Cancer Res Treat.
2024 Jul;56(3):743-750. 10.4143/crt.2023.980.
Phase 1/2a Study of Rivoceranib, a Selective VEGFR-2 Angiogenesis Inhibitor, in Patients with Advanced Solid Tumors
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2University of Utah and Huntsman Cancer Institute, Salt Lake City, UT, USA
- 3Elevar Therapeutics, Fort Lee, NJ, USA
- 4Translational Genomics Research Institute, Phoenix, AZ, USA
- KMID: 2557661
- DOI: http://doi.org/10.4143/crt.2023.980
Abstract
- Purpose
This study aimed to report the results from an early-phase study of rivoceranib, an oral tyrosine kinase inhibitor highly selective for vascular endothelial growth factor receptor 2, in patients with advanced solid tumors.
Materials and Methods
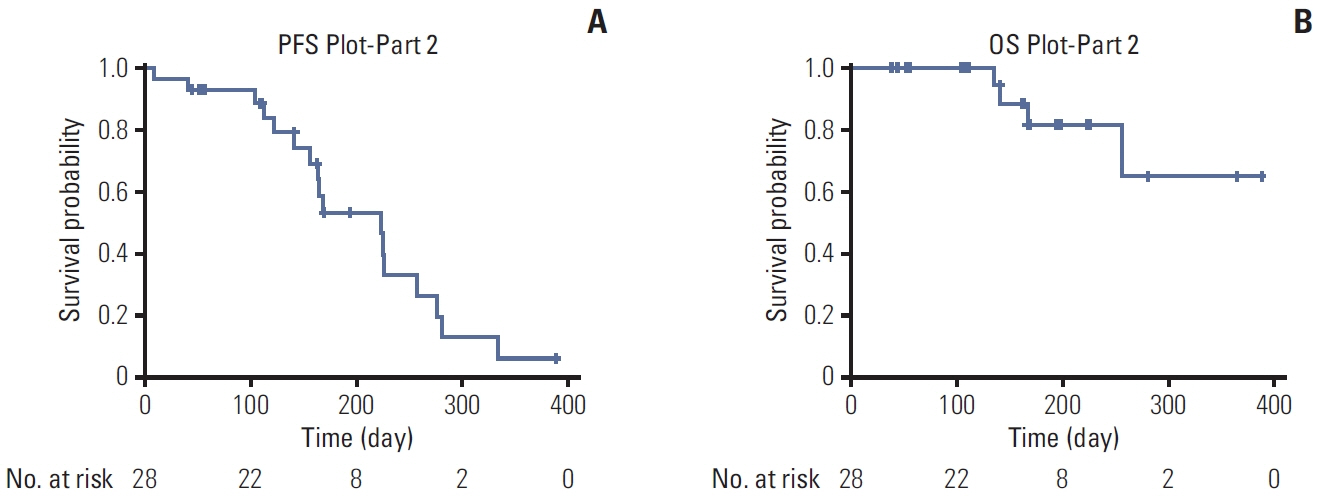
In this open-label, single-arm, dose-escalating, multicenter three-part phase 1/2a trial, patients had advanced solid tumors refractory to conventional therapy. Part 1 evaluated the safety and pharmacokinetics of five ascending once-daily doses of rivoceranib from 81 mg to 685 mg. Part 2 evaluated the safety and antitumor activity of once-daily rivoceranib 685 mg. Part 3 was conducted later, due to lack of maximum tolerated dose determination in part 1, to evaluate the safety and preliminary efficacy of once-daily rivoceranib 805 mg in patients with unresectable or advanced gastric cancer.
Results
A total of 61 patients were enrolled in parts 1 (n=25), 2 (n=30), and 3 (n=6). In parts 1 and 2, patients were white (45.5%) or Asian (54.5%), and 65.6% were male. The most common grade ≥ 3 adverse events were hypertension (32.7%), hyponatremia (10.9%), and hypophosphatemia (10.9%). The objective response rate (ORR) was 15.2%. In part 3, dose-limiting toxicities occurred in two out of six patients: grade 3 febrile neutropenia decreased appetite, and fatigue. The ORR was 33%.
Conclusion
The recommended phase 2 dose of rivoceranib was determined to be 685 mg once daily, which showed adequate efficacy with a manageable safety profile (NCT01497704 and NCT02711969).
Keyword
Figure
Reference
-
References
1. Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer. 2011; 2:1097–105.
Article2. Wang X, Bove AM, Simone G, Ma B. Molecular bases of VEGFR-2-mediated physiological function and pathological role. Front Cell Dev Biol. 2020; 8:599281.
Article3. Niu G, Chen X. Vascular endothelial growth factor as an antiangiogenic target for cancer therapy. Curr Drug Targets. 2010; 11:1000–17.
Article4. Lian L, Li XL, Xu MD, Li XM, Wu MY, Zhang Y, et al. VEGFR2 promotes tumorigenesis and metastasis in a pro-angiogenicindependent way in gastric cancer. BMC Cancer. 2019; 19:183.
Article5. Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014; 383:31–9.
Article6. Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014; 15:78–86.
Article7. Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012; 30:1513–8.
Article8. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014; 15:1224–35.
Article9. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines). Gastric cancer (version 1.2023) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2023 [cited 2023 Dec 20]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf.10. Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016; 34:1448–54.11. Kang YK, Kang WK, Di Bartolomeo M, Chau I, Yoon HH, Cascinu S, et al. Randomized phase III ANGEL study of rivoceranib (apatinib)+best supportive care (BSC) vs placebo+BSC in patients with advanced/metastatic gastric cancer who failed ≥ 2 prior chemotherapy regimens. Ann Oncol. 2019; 30:V877–8.12. Qin S, Chan SL, Gu S, Bai Y, Ren Z, Lin X, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. 2023; 402:1133–46.13. Hanna GJ, Ahn MJ, Muzaffar J, Keam B, Bowles DW, Wong DJ, et al. A phase II trial of rivoceranib, an oral vascular endothelial growth factor receptor 2 inhibitor, for recurrent or metastatic adenoid cystic carcinoma. Clin Cancer Res. 2023; 29:4555–63.
Article14. Eng C, Park CH, Meng X, Jang SH. Phase Ib/II open-label study to evaluate the safety, tolerability, and efficacy of rivoceranib plus trifluridine/tipiracil in patients with previously treated metastatic colorectal cancer. Ann Oncol. 2023; 34(Suppl 2):S453–4.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of Lymphatic Endothelial Growth Factor Receptor in a Murine Model of Oral Squamous Cell Carcinoma
- In vitro evaluation of the antitumor activity of axitinib in canine mammary gland tumor cell lines
- VEGF-VEGFR Signals in Health and Disease
- Immunotherapy in Pediatric Solid Tumors
- Expression of Vascular Endothelial Growth Factor Receptor mRNA in Eutopic and Ectopic Endometrial Tissues of Patients with Endometriosis