Korean J Physiol Pharmacol.
2024 Jul;28(4):323-333. 10.4196/kjpp.2024.28.4.323.
3,3′,4,4′-tetrachlorobiphenyl (PCB77) enhances human Kv1.3 channel currents and alters cytokine production
- Affiliations
-
- 1Department of Physiology, Institute of Bioscience and Biotechnology, Kangwon National University School of Medicine, Chuncheon 24341, Korea
- 2Interdisciplinary Graduate Program in BIT Medical Convergence, Chuncheon 24341, Korea
- 3Department of Biological Sciences and Kangwon Radiation Convergence Research Support Center, Kangwon National University, Chuncheon 24341, Korea
- KMID: 2557204
- DOI: http://doi.org/10.4196/kjpp.2024.28.4.323
Abstract
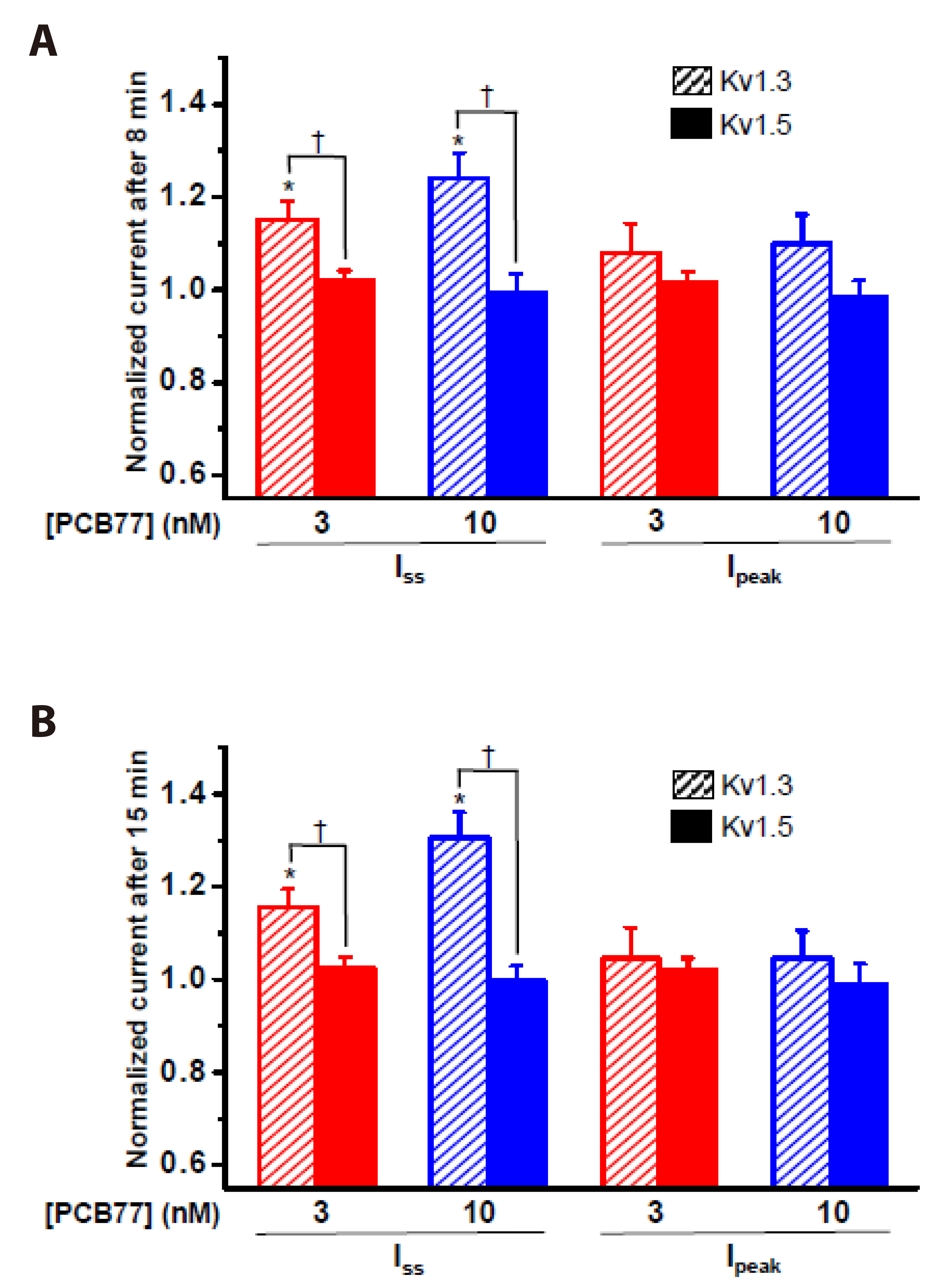
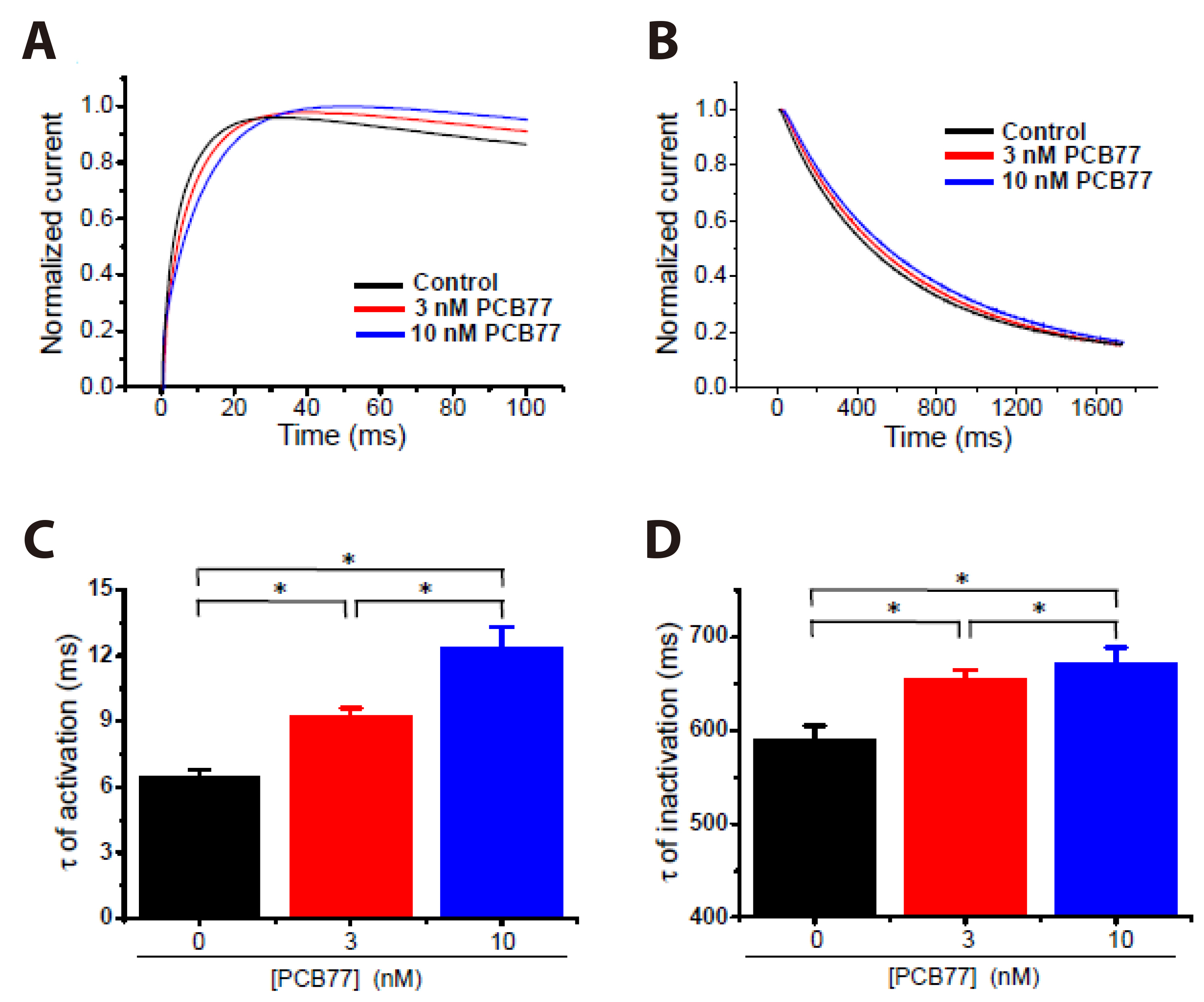
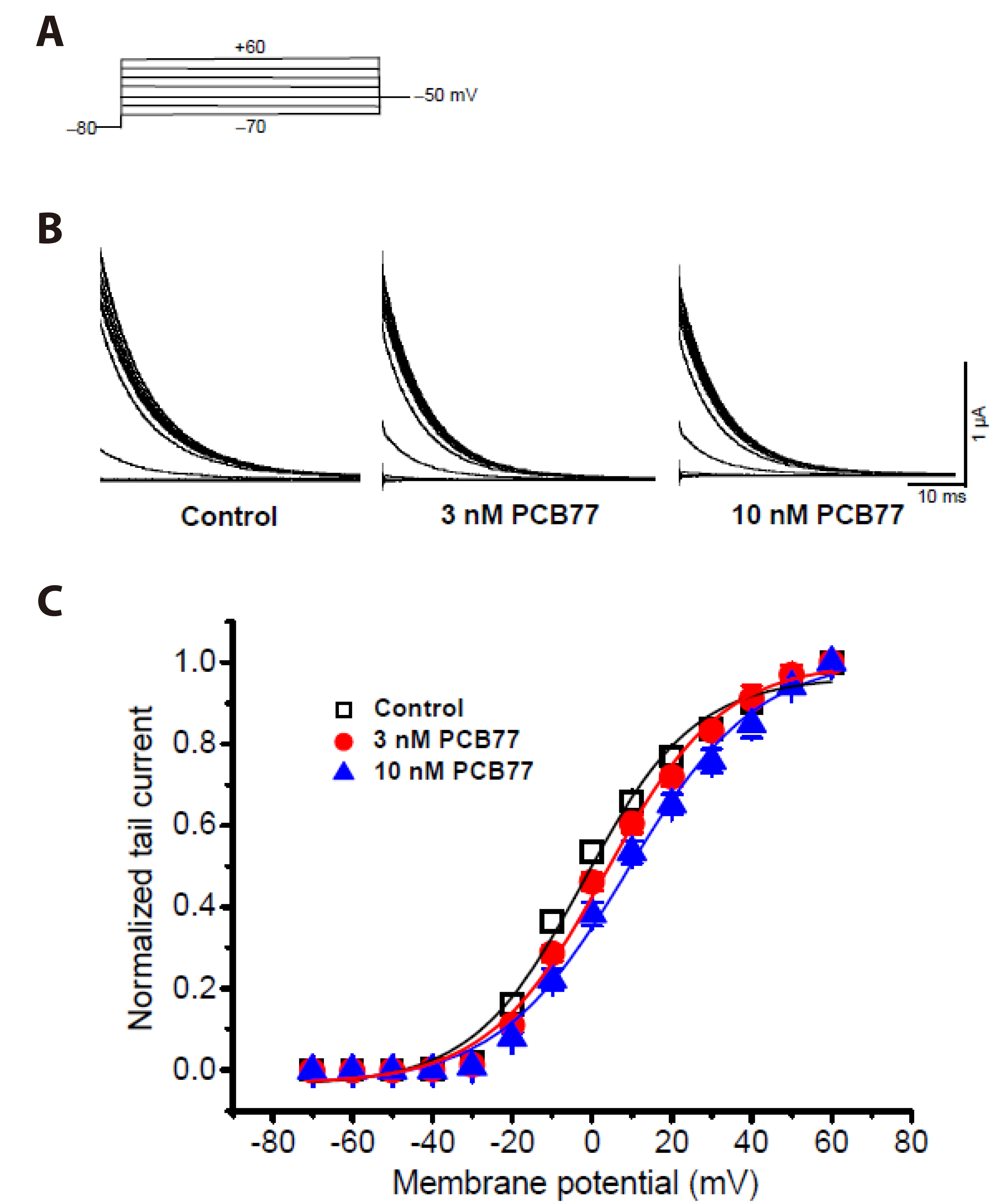
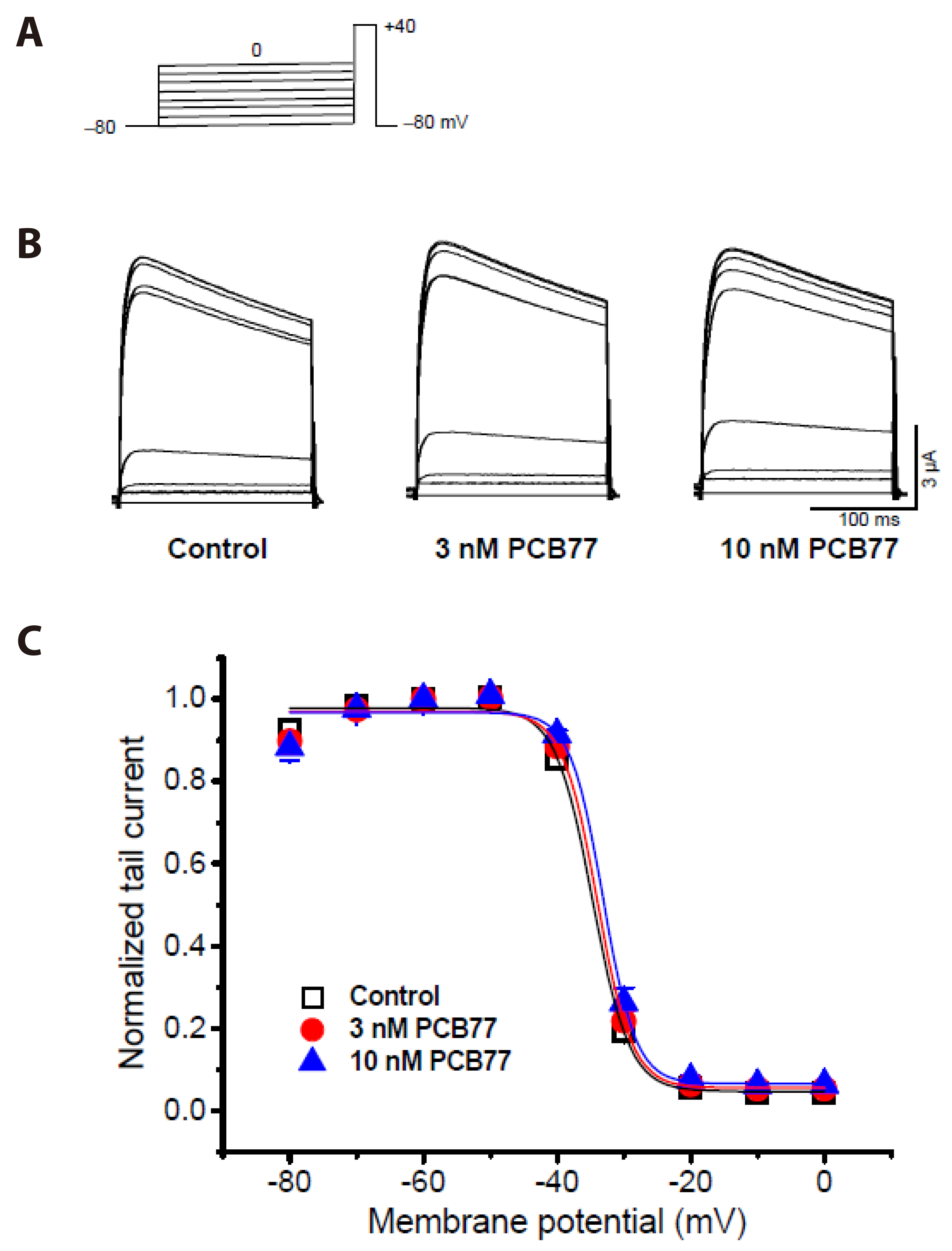
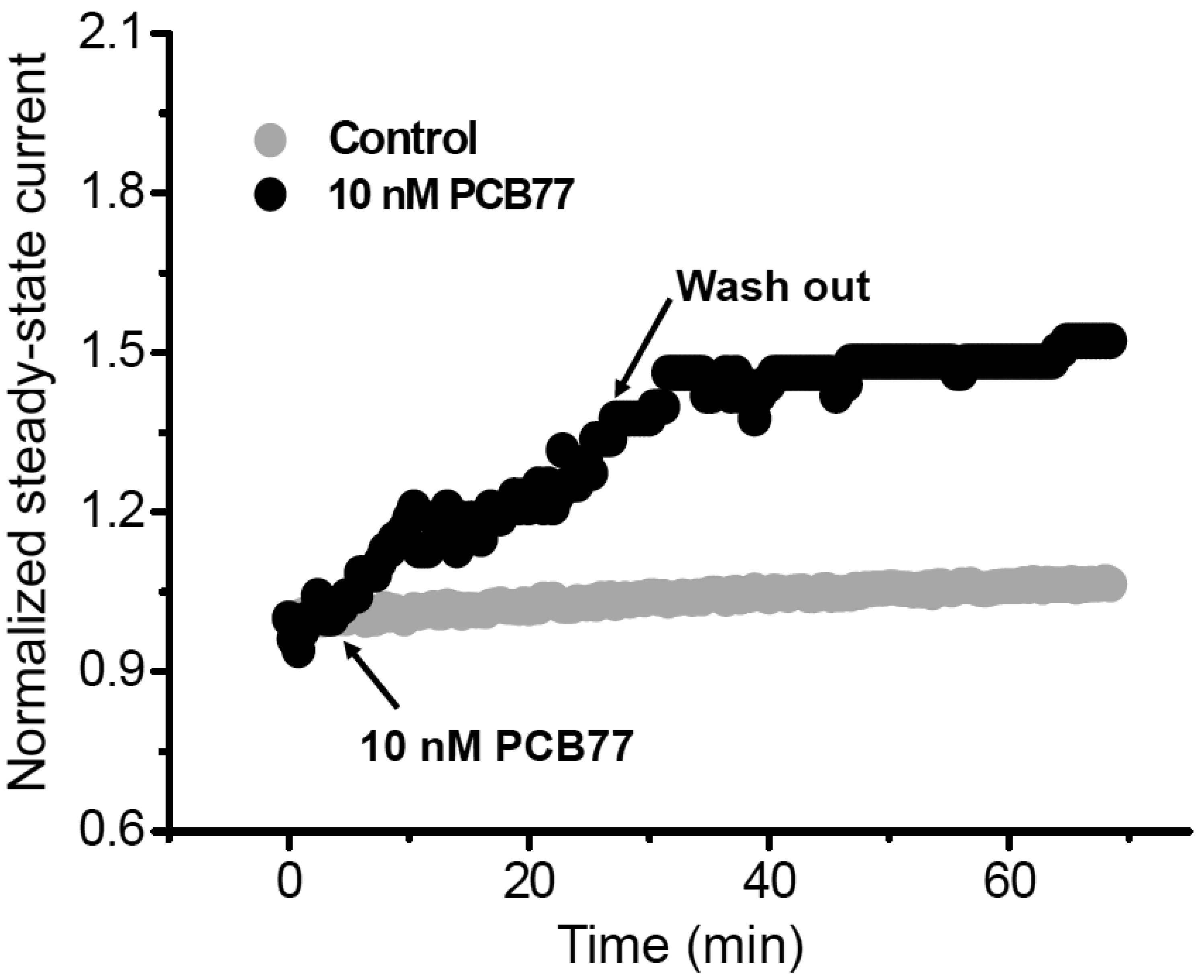
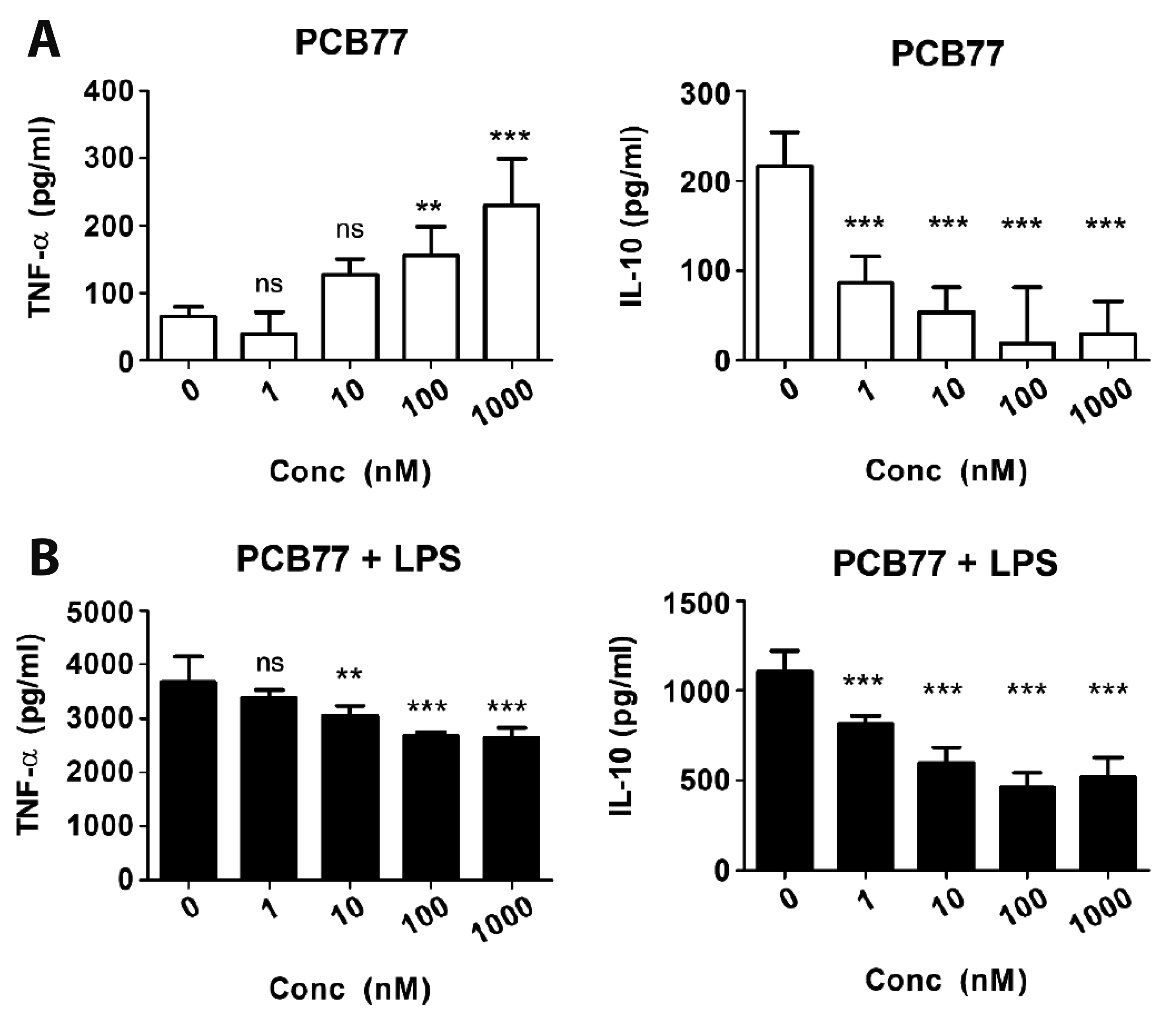
- Polychlorinated biphenyls (PCBs) were once used throughout various industries; however, because of their persistence in the environment, exposure remains a global threat to the environment and human health. The Kv1.3 and Kv1.5 channels have been implicated in the immunotoxicity and cardiotoxicity of PCBs, respectively. We determined whether 3,3′,4,4′-tetrachlorobiphenyl (PCB77), a dioxin-like PCB, alters human Kv1.3 and Kv1.5 currents using the Xenopus oocyte expression system. Exposure to 10 nM PCB77 for 15 min enhanced the Kv1.3 current by approximately 30.6%, whereas PCB77 did not affect the Kv1.5 current at concentrations up to 10 nM. This increase in the Kv1.3 current was associated with slower activation and inactivation kinetics as well as right-shifting of the steady-state activation curve. Pretreatment with PCB77 significantly suppressed tumor necrosis factor-α and interleukin-10 production in lipopolysaccharide-stimulated Raw264.7 macrophages. Overall, these data suggest that acute exposure to trace concentrations of PCB77 impairs immune function, possibly by enhancing Kv1.3 currents.
Keyword
Figure
Reference
-
1. Carpenter DO. 2006; Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev Environ Health. 21:1–23. DOI: 10.1515/REVEH.2006.21.1.1. PMID: 16700427.2. McFarland VA, Clarke JU. 1989; Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: considerations for a congener-specific analysis. Environ Health Perspect. 81:225–239. DOI: 10.1289/ehp.8981225. PMID: 2503374. PMCID: PMC1567542.3. Borja J, Taleon DM, Auresenia J, Gallardo S. 2005; Polychlorinated biphenyls and their biodegradation. Process Biochem. 40:1999–2013. DOI: 10.1016/j.procbio.2004.08.006.4. Jacobson JL, Jacobson SW, Humphrey HE. 1990; Effects of in utero exposure to polychlorinated biphenyls and related contaminants on cognitive functioning in young children. J Pediatr. 116:38–45. DOI: 10.1016/S0022-3476(05)81642-7. PMID: 2104928.5. Carpenter DO. 1998; Polychlorinated biphenyls and human health. Int J Occup Med Environ Health. 11:291–303.6. van den Berg KJ, Zurcher C, Brouwer A. 1988; Effects of 3,4,3',4'-tetrachlorobiphenyl on thyroid function and histology in marmoset monkeys. Toxicol Lett. 41:77–86. DOI: 10.1016/0378-4274(88)90010-0. PMID: 3128898.7. Sánchez-Alonso JA, López-Aparicio P, Recio MN, Pérez-Albarsanz MA. 2003; Apoptosis-mediated neurotoxic potential of a planar (PCB 77) and a nonplanar (PCB 153) polychlorinated biphenyl congeners in neuronal cell cultures. Toxicol Lett. 144:337–349. DOI: 10.1016/S0378-4274(03)00238-8. PMID: 12927351.8. Ramamoorthy K, Gupta MS, Sun G, McDougal A, Safe SH. 1999; 3,3'4,4'-Tetrachlorobiphenyl exhibits antiestrogenic and antitumorigenic activity in the rodent uterus and mammary cells and in human breast cancer cells. Carcinogenesis. 20:115–123. DOI: 10.1093/carcin/20.1.115. PMID: 9934858.9. Silkworth JB, Antrim L, Kaminsky LS. 1984; Correlations between polychlorinated biphenyl immunotoxicity, the aromatic hydrocarbon locus, and liver microsomal enzyme induction in C57BL/6 and DBA/2 mice. Toxicol Appl Pharmacol. 75:156–165. DOI: 10.1016/0041-008X(84)90086-3. PMID: 6431639.10. Yilmaz B, Sandal S, Chen CH, Carpenter DO. 2006; Effects of PCB 52 and PCB 77 on cell viability, [Ca(2+)](i) levels and membrane fluidity in mouse thymocytes. Toxicology. 217:184–193. DOI: 10.1016/j.tox.2005.09.008. PMID: 16233944.11. Kerkvliet NI, Steppan LB, Brauner JA, Deyo JA, Henderson MC, Tomar RS, Buhler DR. 1990; Influence of the Ah locus on the humoral immunotoxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin: evidence for Ah-receptor-dependent and Ah-receptor-independent mechanisms of immunosuppression. Toxicol Appl Pharmacol. 105:26–36. DOI: 10.1016/0041-008X(90)90356-Y. PMID: 2168100.12. Lavoie ET, Grasman KA. 2007; Effects of in ovo exposure to PCBs 126 and 77 on mortality, deformities and post-hatch immune function in chickens. J Toxicol Environ Health A. 70:547–558. DOI: 10.1080/15287390600882226. PMID: 17365608.13. Rudy B. 1988; Diversity and ubiquity of K channels. Neuroscience. 25:729–749. DOI: 10.1016/0306-4522(88)90033-4. PMID: 2457185.14. Felipe A, Vicente R, Villalonga N, Roura-Ferrer M, Martínez-Mármol R, Solé L, Ferreres JC, Condom E. 2006; Potassium channels: new targets in cancer therapy. Cancer Detect Prev. 30:375–385. DOI: 10.1016/j.cdp.2006.06.002. PMID: 16971052.15. Comes N, Bielanska J, Vallejo-Gracia A, Serrano-Albarrás A, Marruecos L, Gómez D, Soler C, Condom E, Hernández-Losa J, Ferreres JC, Felipe A. Ramón Y Cajal S. 2013; The voltage-dependent K(+) channels Kv1.3 and Kv1.5 in human cancer. Front Physiol. 4:283. DOI: 10.3389/fphys.2013.00283. PMID: 24133455. PMCID: PMC3794381.16. Feng J, Wible B, Li GR, Wang Z, Nattel S. 1997; Antisense oligodeoxynucleotides directed against Kv1.5 mRNA specifically inhibit ultrarapid delayed rectifier K+ current in cultured adult human atrial myocytes. Circ Res. 80:572–579. DOI: 10.1161/01.RES.80.4.572. PMID: 9118489.17. Decher N, Pirard B, Bundis F, Peukert S, Baringhaus KH, Busch AE, Steinmeyer K, Sanguinetti MC. 2004; Molecular basis for Kv1.5 channel block: conservation of drug binding sites among voltage-gated K+ channels. J Biol Chem. 279:394–400. DOI: 10.1074/jbc.M307411200. PMID: 14578345.18. Honoré E, Barhanin J, Attali B, Lesage F, Lazdunski M. 1994; External blockade of the major cardiac delayed-rectifier K+ channel (Kv1.5) by polyunsaturated fatty acids. Proc Natl Acad Sci U S A. 91:1937–1941. DOI: 10.1073/pnas.91.5.1937. PMID: 8127910. PMCID: PMC43279.19. Olson TM, Alekseev AE, Liu XK, Park S, Zingman LV, Bienengraeber M, Sattiraju S, Ballew JD, Jahangir A, Terzic A. 2006; Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 15:2185–2191. DOI: 10.1093/hmg/ddl143. PMID: 16772329.20. Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. 2005; International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev. 57:463–472. DOI: 10.1124/pr.57.4.9. PMID: 16382103.21. Pérez-Verdaguer M, Capera J, Serrano-Novillo C, Estadella I, Sastre D, Felipe A. 2016; The voltage-gated potassium channel Kv1.3 is a promising multitherapeutic target against human pathologies. Expert Opin Ther Targets. 20:577–591. DOI: 10.1517/14728222.2016.1112792. PMID: 26634786.22. Pérez-García MT, Cidad P, López-López JR. 2018; The secret life of ion channels: Kv1.3 potassium channels and proliferation. Am J Physiol Cell Physiol. 314:C27–C42. DOI: 10.1152/ajpcell.00136.2017. PMID: 28931540.23. Wulff H, Calabresi PA, Allie R, Yun S, Pennington M, Beeton C, Chandy KG. 2003; The voltage-gated Kv1.3 K(+) channel in effector memory T cells as new target for MS. J Clin Invest. 111:1703–1713. Erratum in: J Clin Invest. 2003;112:298. DOI: 10.1172/JCI16921. PMID: 12782673. PMCID: PMC156104.24. Hu L, Pennington M, Jiang Q, Whartenby KA, Calabresi PA. 2007; Characterization of the functional properties of the voltage-gated potassium channel Kv1.3 in human CD4+ T lymphocytes. J Immunol. 179:4563–4570. DOI: 10.4049/jimmunol.179.7.4563. PMID: 17878353.25. Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, Sanchez M, Giangiacomo K, Reuben JP, Smith AB 3rd, Kaczorowski GJ, Garcia ML. 1994; Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 33:5819–5828. DOI: 10.1021/bi00185a021. PMID: 7514038.26. Cahalan MD, Chandy KG. 1997; Ion channels in the immune system as targets for immunosuppression. Curr Opin Biotechnol. 8:749–756. DOI: 10.1016/S0958-1669(97)80130-9. PMID: 9425667.27. Zhu H, Yan L, Gu J, Hao W, Cao J. 2015; Kv1.3 channel blockade enhances the phagocytic function of RAW264.7 macrophages. Sci China Life Sci. 58:867–875. DOI: 10.1007/s11427-015-4915-3. PMID: 26354506.28. Di Lucente J, Nguyen HM, Wulff H, Jin LW, Maezawa I. 2018; The voltage-gated potassium channel Kv1.3 is required for microglial pro-inflammatory activation in vivo. Glia. 66:1881–1895. DOI: 10.1002/glia.23457. PMID: 30043400. PMCID: PMC6185766.29. Jo SH, Hong HK, Chong SH, Won KH, Jung SJ, Choe H. 2008; Clomipramine block of the hERG K+ channel: accessibility to F656 and Y652. Eur J Pharmacol. 592:19–25. DOI: 10.1016/j.ejphar.2008.06.094. PMID: 18634780.30. Lee HJ, Kang SJ, Woo Y, Hahn TW, Ko HJ, Jung YJ. 2020; TLR7 stimulation with imiquimod induces selective autophagy and controls Mycobacterium tuberculosis growth in mouse macrophages. Front Microbiol. 11:1684. DOI: 10.3389/fmicb.2020.01684. PMID: 32765474. PMCID: PMC7380068.31. Mohammad HMF, El-Baz AA, Mahmoud OM, Khalil S, Atta R, Imbaby S. 2023; Protective effects of evening primrose oil on behavioral activities, nigral microglia and histopathological changes in a rat model of rotenone-induced parkinsonism. J Chem Neuroanat. 127:102206. DOI: 10.1016/j.jchemneu.2022.102206. PMID: 36464068.32. Serrano-Albarrás A, Estadella I, Cirera-Rocosa S, Navarro-Pérez M, Felipe A. 2018; Kv1.3: a multifunctional channel with many pathological implications. Expert Opin Ther Targets. 22:101–105. DOI: 10.1080/14728222.2017.1420170. PMID: 29258373.33. Teisseyre A, Palko-Labuz A, oda-Pomianek K Sr, Michalak K. 2019; Voltage-gated potassium channel Kv1.3 as a target in therapy of cancer. Front Oncol. 9:933. DOI: 10.3389/fonc.2019.00933. PMID: 31612103. PMCID: PMC6769076.34. Kang JA, Park SH, Jeong SP, Han MH, Lee CR, Lee KM, Kim N, Song MR, Choi M, Ye M, Jung G, Lee WW, Eom SH, Park CS, Park SG. 2016; Epigenetic regulation of Kcna3-encoding Kv1.3 potassium channel by cereblon contributes to regulation of CD4+ T-cell activation. Proc Natl Acad Sci U S A. 113:8771–8776. DOI: 10.1073/pnas.1502166113. PMID: 27439875. PMCID: PMC4978309.35. Kim JH, Hwang S, Jo SH. 2021; Non-dioxin-like polychlorinated biphenyl 19 has distinct effects on human Kv1.3 and Kv1.5 channels. Toxicol Appl Pharmacol. 411:115365. DOI: 10.1016/j.taap.2020.115365. PMID: 33316272.36. Kim JH, Hwang S, Park SI, Jo SH. 2019; Effects of 3, 3, 4, 4, 5-pentachlorobiphenyl on human Kv1.3 and Kv1.5 channels. Int J Oral Biol. 44:115–123. DOI: 10.11620/IJOB.2019.44.3.115.37. Poland A, Knutson JC. 1982; 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 22:517–554. DOI: 10.1146/annurev.pa.22.040182.002505. PMID: 6282188.38. Dean KM, Marcell AM, Baltos LD, Carro T, Bohannon MEB, Ottinger MA. 2019; Comparative lethality of in ovo exposure to PCB 126, PCB 77, and 2 environmentally relevant PCB mixtures in Japanese Quail (Coturnix japonica). Environ Toxicol Chem. 38:2637–2650. DOI: 10.1002/etc.4578. PMID: 31436847.39. Beck H, Breuer EM, Droß A, Mathar W. 1990; Residues of PCDDs, PCDFs, PCbs and other organochlorine compounds in harbour seals and harbour porpoise. Chemosphere. 20:1027–1034. DOI: 10.1016/0045-6535(90)90216-G.40. Zhang Y, Wise JP, Holford TR, Xie H, Boyle P, Zahm SH, Rusiecki J, Zou K, Zhang B, Zhu Y, Owens PH, Zheng T. 2004; Serum polychlorinated biphenyls, cytochrome P-450 1A1 polymorphisms, and risk of breast cancer in Connecticut women. Am J Epidemiol. 160:1177–1183. DOI: 10.1093/aje/kwh346. PMID: 15583370.41. Choi SY, Koh YS, Jo SH. 2005; Inhibition of human ether-a-go-go-related gene K+ channel and IKr of guinea pig cardiomyocytes by antipsychotic drug trifluoperazine. J Pharmacol Exp Ther. 313:888–895. DOI: 10.1124/jpet.104.080853. PMID: 15722405.42. Serrano-Novillo C, Capera J, Colomer-Molera M, Condom E, Ferreres JC, Felipe A. 2019; Implication of voltage-gated potassium channels in neoplastic cell proliferation. Cancers (Basel). 11:287. DOI: 10.3390/cancers11030287. PMID: 30823672. PMCID: PMC6468671.43. Lewis RS, Cahalan MD. 1995; Potassium and calcium channels in lymphocytes. Annu Rev Immunol. 13:623–653. DOI: 10.1146/annurev.iy.13.040195.003203. PMID: 7612237.44. Kan XH, Gao HQ, Ma ZY, Liu L, Ling MY, Wang YY. 2016; Kv1.3 potassium channel mediates macrophage migration in atherosclerosis by regulating ERK activity. Arch Biochem Biophys. 591:150–156. DOI: 10.1016/j.abb.2015.12.013. PMID: 26748289.45. Wulff H, Castle NA, Pardo LA. 2009; Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 8:982–1001. DOI: 10.1038/nrd2983. PMID: 19949402. PMCID: PMC2790170.46. Wang X, Li G, Guo J, Zhang Z, Zhang S, Zhu Y, Cheng J, Yu L, Ji Y, Tao J. 2020; Kv1.3 channel as a key therapeutic target for neuroinflammatory diseases: state of the art and beyond. Front Neurosci. 13:1393. Erratum in: Front Neurosci. 2020;14:163. DOI: 10.3389/fnins.2019.01393. PMID: 31992966. PMCID: PMC6971160.47. Villalonga N, David M, Bielanska J, Vicente R, Comes N, Valenzuela C, Felipe A. 2010; Immunomodulation of voltage-dependent K+ channels in macrophages: molecular and biophysical consequences. J Gen Physiol. 135:135–147. DOI: 10.1085/jgp.200910334. PMID: 20100893. PMCID: PMC2812499.48. Vaeth M, Feske S. 2018; Ion channelopathies of the immune system. Curr Opin Immunol. 52:39–50. DOI: 10.1016/j.coi.2018.03.021. PMID: 29635109. PMCID: PMC6004246.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The antidiabetic drug rosiglitazone blocks Kv1.5 potassium channels in an open state
- Direct Block of Cloned K+ Channels, Kv1.5 and Kv1.3, by Cyclosporin A, Independent of Calcineurin Inhibition
- Blockade of Kv1.5 by paroxetine, an antidepressant drug
- Open channel block of Kv1.4 potassium channels by aripiprazole
- Blockade of Kv1.5 channels by the antidepressant drug sertraline