Korean J Physiol Pharmacol.
2020 Nov;24(6):545-553. 10.4196/kjpp.2020.24.6.545.
Open channel block of Kv1.4 potassium channels by aripiprazole
- Affiliations
-
- 1Department of Physiology,College of Medicine, The Catholic University of Korea, Seoul 06591, Korea
- 2College of Pharmacy, Integrated Research Institute of Pharmaceutical, The Catholic University of Korea, Bucheon 14662, Korea
- 3Department of Catholic Neuroscience Institute, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea
- KMID: 2507736
- DOI: http://doi.org/10.4196/kjpp.2020.24.6.545
Abstract
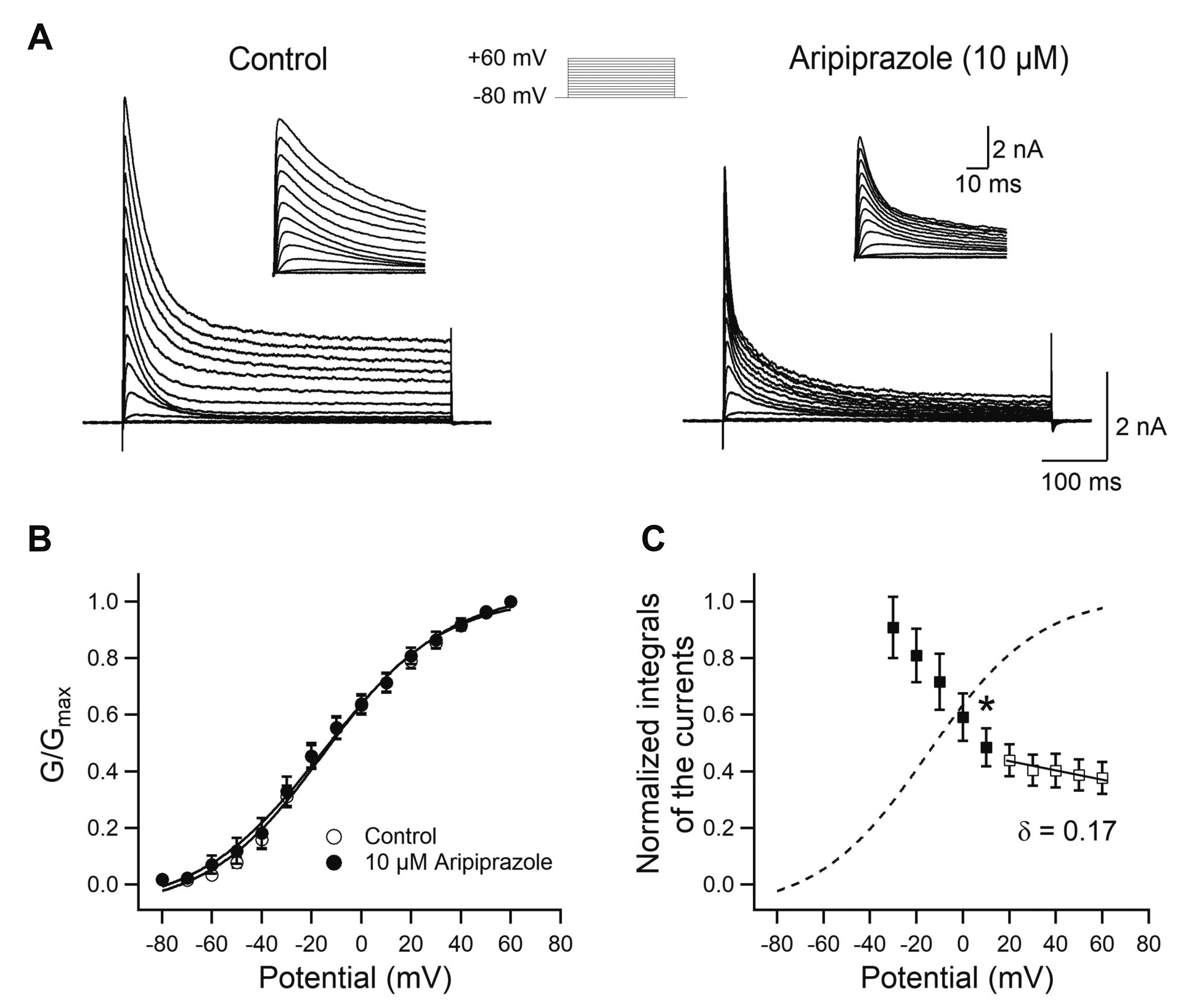
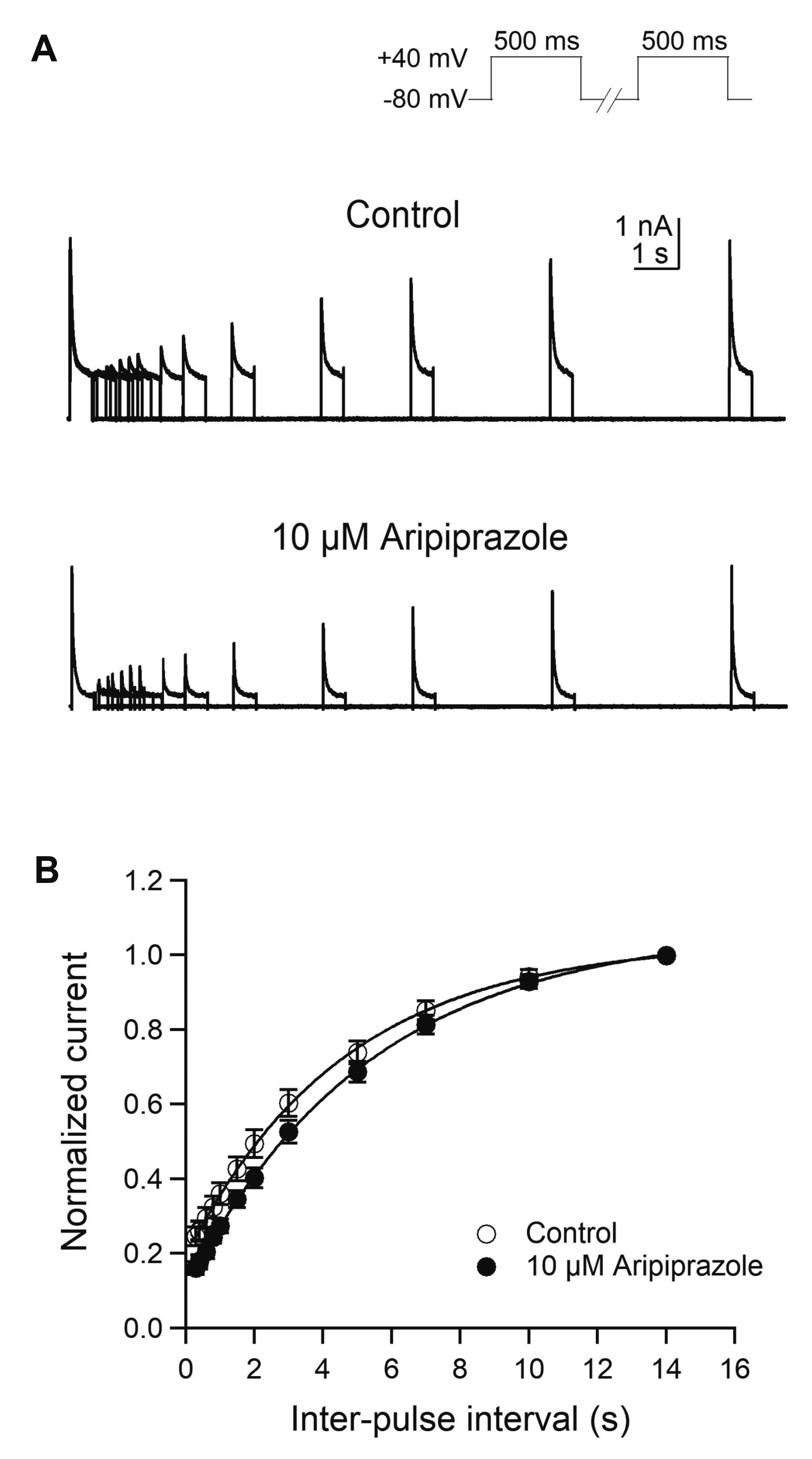
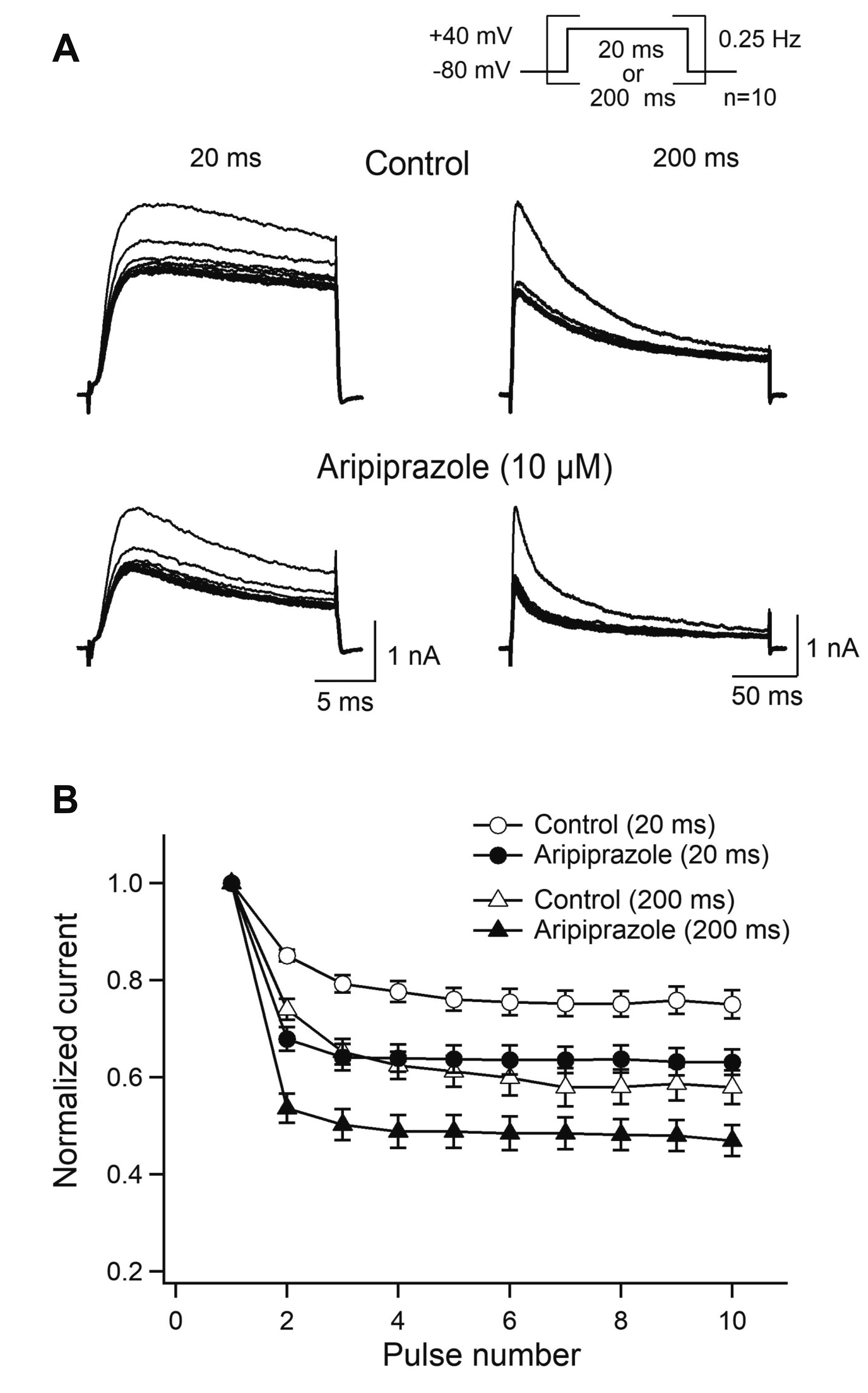
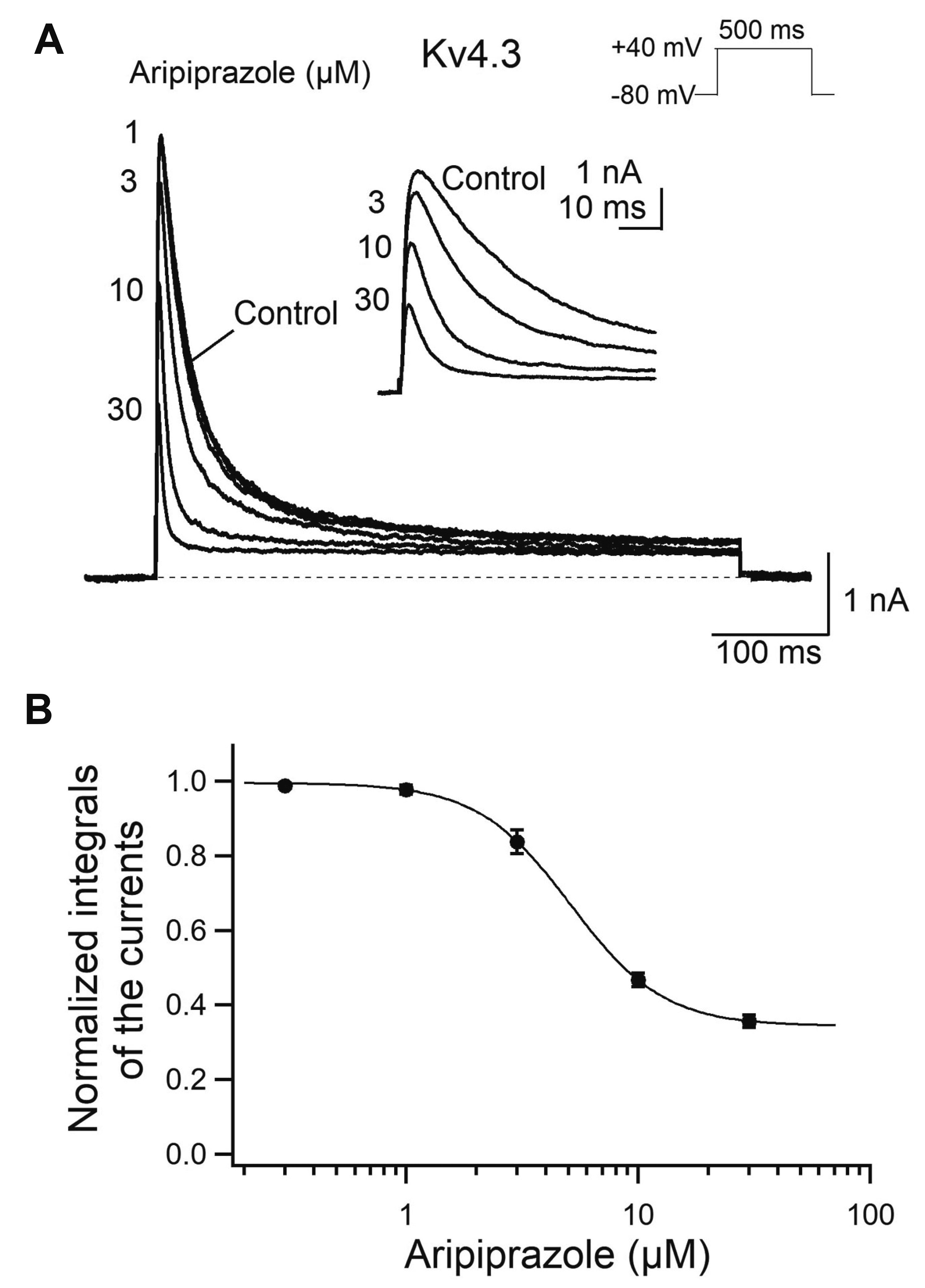
- Aripiprazole is a quinolinone derivative approved as an atypical antipsychotic drug for the treatment of schizophrenia and bipolar disorder. It acts as with partial agonist activities at the dopamine D2 receptors. Although it is known to be relatively safe for patients with cardiac ailments, less is known about the effect of aripiprazole on voltage-gated ion channels such as transient A-type K+ channels, which are important for the repolarization of cardiac and neuronal action potentials. Here, we investigated the effects of aripiprazole on Kv1.4 currents expressed in HEK293 cells using a whole-cell patch-clamp technique. Aripiprazole blocked Kv1.4 channels in a concentration-dependent manner with an IC50 value of 4.4 μM and a Hill coefficient of 2.5. Aripiprazole also accelerated the activation (time-to-peak) and inactivation kinetics. Aripiprazole induced a voltage-dependent (δ = 0.17) inhibition, which was use-dependent with successive pulses on Kv1.4 currents without altering the time course of recovery from inactivation. Dehydroaripiprazole, an active metabolite of aripiprazole, inhibited Kv1.4 with an IC50 value of 6.3 μM (p < 0.05 compared with aripiprazole) with a Hill coefficient of 2.0. Furthermore, aripiprazole inhibited Kv4.3 currents to a similar extent in a concentration-dependent manner with an IC50 value of 4.9 μM and a Hill coefficient of 2.3. Thus, our results indicate that aripiprazole blocked Kv1.4 by preferentially binding to the open state of the channels.
Keyword
Figure
Cited by 1 articles
-
The antidiabetic drug rosiglitazone blocks Kv1.5 potassium channels in an open state
Hyang Mi Lee, Sang June Hahn, Bok Hee Choi
Korean J Physiol Pharmacol. 2022;26(2):135-144. doi: 10.4196/kjpp.2022.26.2.135.
Reference
-
1. Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, Yocca FD, Molinoff PB. 2002; Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 302:381–389. DOI: 10.1124/jpet.102.033175. PMID: 12065741.
Article2. Urban JD, Vargas GA, von Zastrow M, Mailman RB. 2007; Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signaling pathways. Neuropsychopharmacology. 32:67–77. DOI: 10.1038/sj.npp.1301071. PMID: 16554739.
Article3. Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R. 2003; Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 28:1400–1411. DOI: 10.1038/sj.npp.1300203. PMID: 12784105.
Article4. Kirschbaum KM, Müller MJ, Malevani J, Mobascher A, Burchardt C, Piel M, Hiemke C. 2008; Serum levels of aripiprazole and dehydroaripiprazole, clinical response and side effects. World J Biol Psychiatry. 9:212–218. DOI: 10.1080/15622970701361255. PMID: 17853280.
Article5. Stahl SM. 2001; Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 2: illustrating their mechanism of action. J Clin Psychiatry. 62:923–924. DOI: 10.4088/JCP.v62n1201. PMID: 11780870.
Article6. Swainston Harrison T, Perry CM. 2004; Aripiprazole: a review of its use in schizophrenia and schizoaffective disorder. Drugs. 64:1715–1736. DOI: 10.2165/00003495-200464150-00010. PMID: 15257633.7. Torgovnick J, Sethi NK, Arsura E. 2008; Aripiprazole-induced orthostatic hypotension and cardiac arrhythmia. Psychiatry Clin Neurosci. 62:485. DOI: 10.1111/j.1440-1819.2008.01833.x. PMID: 18778452.8. Shao Q, Quan W, Jia X, Chen J, Ma S, Zhang X. 2015; Severe arrhythmia induced by orally disintegrating aripiprazole tablets (BosiqingⓇ): a case report. Neuropsychiatr Dis Treat. 11:3019–3021. DOI: 10.2147/NDT.S91771. PMID: 26677328.9. Nelson S, Leung JG. 2013; Torsades de pointes after administration of low-dose aripiprazole. Ann Pharmacother. 47:e11. DOI: 10.1345/aph.1R387. PMID: 23362038.
Article10. Naguy A. 2016; Aripiprazole cardiosafety: is it overestimated? J Family Med Prim Care. 5:736–737. DOI: 10.4103/2249-4863.197283. PMID: 28217624. PMCID: PMC5290802.
Article11. Huang XP, Mangano T, Hufeisen S, Setola V, Roth BL. 2010; Identification of human Ether-à-go-go related gene modulators by three screening platforms in an academic drug-discovery setting. Assay Drug Dev Technol. 8:727–742. DOI: 10.1089/adt.2010.0331. PMID: 21158687.12. Serôdio P, Vega-Saenz de Miera E, Rudy B. 1996; Cloning of a novel component of A-type K+ channels operating at subthreshold potentials with unique expression in heart and brain. J Neurophysiol. 75:2174–2179. DOI: 10.1152/jn.1996.75.5.2174. PMID: 8734615.13. Ohya S, Tanaka M, Oku T, Asai Y, Watanabe M, Giles WR, Imaizumi Y. 1997; Molecular cloning and tissue distribution of an alternatively spliced variant of an A-type K+ channel alpha-subunit, Kv4.3 in the rat. FEBS Lett. 420:47–53. DOI: 10.1016/S0014-5793(97)01483-X. PMID: 9450548.14. O'Donovan B, Adeluyi A, Anderson EL, Cole RD, Turner JR, Ortinski PI. 2019; Altered gating of Kv1.4 in the nucleus accumbens suppresses motivation for reward. Elife. 8:e47870. DOI: 10.7554/eLife.47870. PMID: 31487241. PMCID: PMC6728144.15. Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. 2001; Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A. 98:13373–13378. DOI: 10.1073/pnas.231376298. PMID: 11698689. PMCID: PMC60878.
Article16. Guo W, Li H, London B, Nerbonne JM. 2000; Functional consequences of elimination of i(to,f) and i(to,s): early afterdepolarizations, atrioventricular block, and ventricular arrhythmias in mice lacking Kv1.4 and expressing a dominant-negative Kv4 alpha subunit. Circ Res. 87:73–79. DOI: 10.1161/01.RES.87.1.73. PMID: 10884375.17. Ahn HS, Kim SE, Jang HJ, Kim MJ, Rhie DJ, Yoon SH, Jo YH, Kim MS, Sung KW, Hahn SJ. 2006; Interaction of riluzole with the closed inactivated state of Kv4.3 channels. J Pharmacol Exp Ther. 319:323–331. DOI: 10.1124/jpet.106.106724. PMID: 16815868.
Article18. Chae YJ, Kim DH, Lee HJ, Sung KW, Kwon OJ, Hahn SJ. 2015; Raloxifene inhibits cloned Kv4.3 channels in an estrogen receptor-independent manner. Pflugers Arch. 467:1663–1676. DOI: 10.1007/s00424-014-1602-3. PMID: 25231973.
Article19. Woodhull AM. 1973; Ionic blockage of sodium channels in nerve. J Gen Physiol. 61:687–708. DOI: 10.1085/jgp.61.6.687. PMID: 4541078. PMCID: PMC2203489.
Article20. Choi BH, Choi JS, Ahn HS, Kim MJ, Rhie DJ, Yoon SH, Min DS, Jo YH, Kim MS, Hahn SJ. 2003; Fluoxetine blocks cloned neuronal A-type K+ channels Kv1.4. Neuroreport. 14:2451–2455. DOI: 10.1097/00001756-200312190-00032. PMID: 14663209.
Article21. Weiss JN. 1997; The Hill equation revisited: uses and misuses. FASEB J. 11:835–841. DOI: 10.1096/fasebj.11.11.9285481. PMID: 9285481.
Article22. Castle NA. 1990; Bupivacaine inhibits the transient outward K+ current but not the inward rectifier in rat ventricular myocytes. J Pharmacol Exp Ther. 255:1038–1046. PMID: 2262891.23. Wang Z, Fermini B, Nattel S. 1995; Effects of flecainide, quinidine, and 4-aminopyridine on transient outward and ultrarapid delayed rectifier currents in human atrial myocytes. J Pharmacol Exp Ther. 272:184–196. PMID: 7815332.24. Wood MD, Scott C, Clarke K, Westaway J, Davies CH, Reavill C, Hill M, Rourke C, Newson M, Jones DN, Forbes IT, Gribble A. 2006; Aripiprazole and its human metabolite are partial agonists at the human dopamine D2 receptor, but the rodent metabolite displays antagonist properties. Eur J Pharmacol. 546:88–94. DOI: 10.1016/j.ejphar.2006.07.008. PMID: 16925992.
Article25. Kim SE, Ahn HS, Choi BH, Jang HJ, Kim MJ, Rhie DJ, Yoon SH, Jo YH, Kim MS, Sung KW, Hahn SJ. 2007; Open channel block of A-type, kv4.3, and delayed rectifier K+ channels, Kv1.3 and Kv3.1, by sibutramine. J Pharmacol Exp Ther. 321:753–762. DOI: 10.1124/jpet.106.117747. PMID: 17312186.
Article26. Petersen KR, Nerbonne JM. 1999; Expression environment determines K+ current properties: Kv1 and Kv4 alpha-subunit-induced K+ currents in mammalian cell lines and cardiac myocytes. Pflugers Arch. 437:381–392. DOI: 10.1007/s004240050792. PMID: 9914394.27. Roeper J, Lorra C, Pongs O. 1997; Frequency-dependent inactivation of mammalian A-type K+ channel KV1.4 regulated by Ca2+/calmodulin-dependent protein kinase. J Neurosci. 17:3379–3391. DOI: 10.1523/JNEUROSCI.17-10-03379.1997. PMID: 9133364. PMCID: PMC6573714.28. Wissmann R, Bildl W, Oliver D, Beyermann M, Kalbitzer HR, Bentrop D, Fakler B. 2003; Solution structure and function of the "tandem inactivation domain" of the neuronal A-type potassium channel Kv1.4. J Biol Chem. 278:16142–16150. DOI: 10.1074/jbc.M210191200. PMID: 12590144.
Article29. Ishii K, Nakamura Y, Taira N. Kondoh Si. 1997; A mammalian transient type K+ channel, rat Kv1.4, has two potential domains that could produce rapid inactivation. J Biol Chem. 272:19333–19338. DOI: 10.1074/jbc.272.31.19333. PMID: 9235930.30. Ruppersberg JP, Frank R, Pongs O, Stocker M. 1991; Cloned neuronal IK(A) channels reopen during recovery from inactivation. Nature. 353:657–660. DOI: 10.1038/353657a0. PMID: 1922383.
Article31. Lopez GA, Jan YN, Jan LY. 1994; Evidence that the S6 segment of the Shaker voltage-gated K+ channel comprises part of the pore. Nature. 367:179–182. DOI: 10.1038/367179a0. PMID: 8114915.
Article32. Bett GC, Rasmusson RL. 2004; Inactivation and recovery in Kv1.4 K+ channels: lipophilic interactions at the intracellular mouth of the pore. J Physiol. 556(Pt 1):109–120. DOI: 10.1113/jphysiol.2003.055012. PMID: 14608006. PMCID: PMC1664896.33. Madhav NVS, Ojha A, Jaiswal V. 2018; A smart approach for delivery of aripiprazole via oro-soft palatal mucosal route for improved therapeutic efficacy. Braz J Pharm Sci. 54:e17382. DOI: 10.1590/s2175-97902018000317382.
Article34. Lee HM, Hahn SJ, Choi BH. 2016; Blockade of Kv1.5 channels by the antidepressant drug sertraline. Korean J Physiol Pharmacol. 20:193–200. DOI: 10.4196/kjpp.2016.20.2.193. PMID: 26937216. PMCID: PMC4770110.
Article35. Tytgat J, Maertens C, Daenens P. 1997; Effect of fluoxetine on a neuronal, voltage-dependent potassium channel (Kv1.1). Br J Pharmacol. 122:1417–1424. DOI: 10.1038/sj.bjp.0701545. PMID: 9421290. PMCID: PMC1565099.
Article36. Choi JS, Hahn SJ, Rhie DJ, Yoon SH, Jo YH, Kim MS. 1999; Mechanism of fluoxetine block of cloned voltage-activated potassium channel Kv1.3. J Pharmacol Exp Ther. 291:1–6. PMID: 10490879.37. Sparshatt A, Taylor D, Patel MX, Kapur S. 2010; A systematic review of aripiprazole--dose, plasma concentration, receptor occupancy, and response: implications for therapeutic drug monitoring. J Clin Psychiatry. 71:1447–1456. DOI: 10.4088/JCP.09r05060gre. PMID: 20584524.38. Citrome L, Josiassen R, Bark N, Salazar DE, Mallikaarjun S. 2005; Pharmacokinetics of aripiprazole and concomitant lithium and valproate. J Clin Pharmacol. 45:89–93. DOI: 10.1177/0091270004269870. PMID: 15601809.
Article39. Boulton DW, Kollia G, Mallikaarjun S, Komoroski B, Sharma A, Kovalick LJ, Reeves RA. 2008; Pharmacokinetics and tolerability of intramuscular, oral and intravenous aripiprazole in healthy subjects and in patients with schizophrenia. Clin Pharmacokinet. 47:475–485. DOI: 10.2165/00003088-200847070-00004. PMID: 18563956.40. Sheng M, Tsaur ML, Jan YN, Jan LY. 1992; Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 9:271–284. DOI: 10.1016/0896-6273(92)90166-B. PMID: 1497894.
Article41. Zemel BM, Ritter DM, Covarrubias M, Muqeem T. 2018; A-Type KV channels in dorsal root ganglion neurons: diversity, function, and dysfunction. Front Mol Neurosci. 11:253. DOI: 10.3389/fnmol.2018.00253. PMID: 30127716. PMCID: PMC6088260.
Article42. Norris AJ, Nerbonne JM. 2010; Molecular dissection of I(A) in cortical pyramidal neurons reveals three distinct components encoded by Kv4.2, Kv4.3, and Kv1.4 alpha-subunits. J Neurosci. 30:5092–5101. DOI: 10.1523/JNEUROSCI.5890-09.2010. PMID: 20371829. PMCID: PMC2862390.43. Burkhalter A, Gonchar Y, Mellor RL, Nerbonne JM. 2006; Differential expression of IA channel subunits Kv4.2 and Kv4.3 in mouse visual cortical neurons and synapses. J Neurosci. 26:12274–12282. DOI: 10.1523/JNEUROSCI.2599-06.2006. PMID: 17122053.44. Hoffman DA, Magee JC, Colbert CM, Johnston D. 1997; K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 387:869–875. DOI: 10.1038/43119. PMID: 9202119.
Article45. Patel SP, Campbell DL. 2005; Transient outward potassium current, 'Ito', phenotypes in the mammalian left ventricle: underlying molecular, cellular and biophysical mechanisms. J Physiol. 569(Pt 1):7–39. DOI: 10.1113/jphysiol.2005.086223. PMID: 15831535. PMCID: PMC1464208.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The antidiabetic drug rosiglitazone blocks Kv1.5 potassium channels in an open state
- Effects of 3,3′,4,4′,5-pentachlorobiphenyl on human Kv1.3 and Kv1.5 channels
- Direct Block of Cloned K+ Channels, Kv1.5 and Kv1.3, by Cyclosporin A, Independent of Calcineurin Inhibition
- Regulation of Antiarrhythmic Drug Propafenone Effects on the C-type KV1.4 Potassium Channel by PHo and K+
- K+ channel