Blockade of Kv1.5 by paroxetine, an antidepressant drug
- Affiliations
-
- 1Department of Pharmacology, Institute for Medical Sciences, Chonbuk National University Medical School, Jeonju 54097, Korea. bhchoi@jbnu.ac.kr
- 2Department of Physiology, Medical Research Center, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- KMID: 2150476
- DOI: http://doi.org/10.4196/kjpp.2016.20.1.75
Abstract
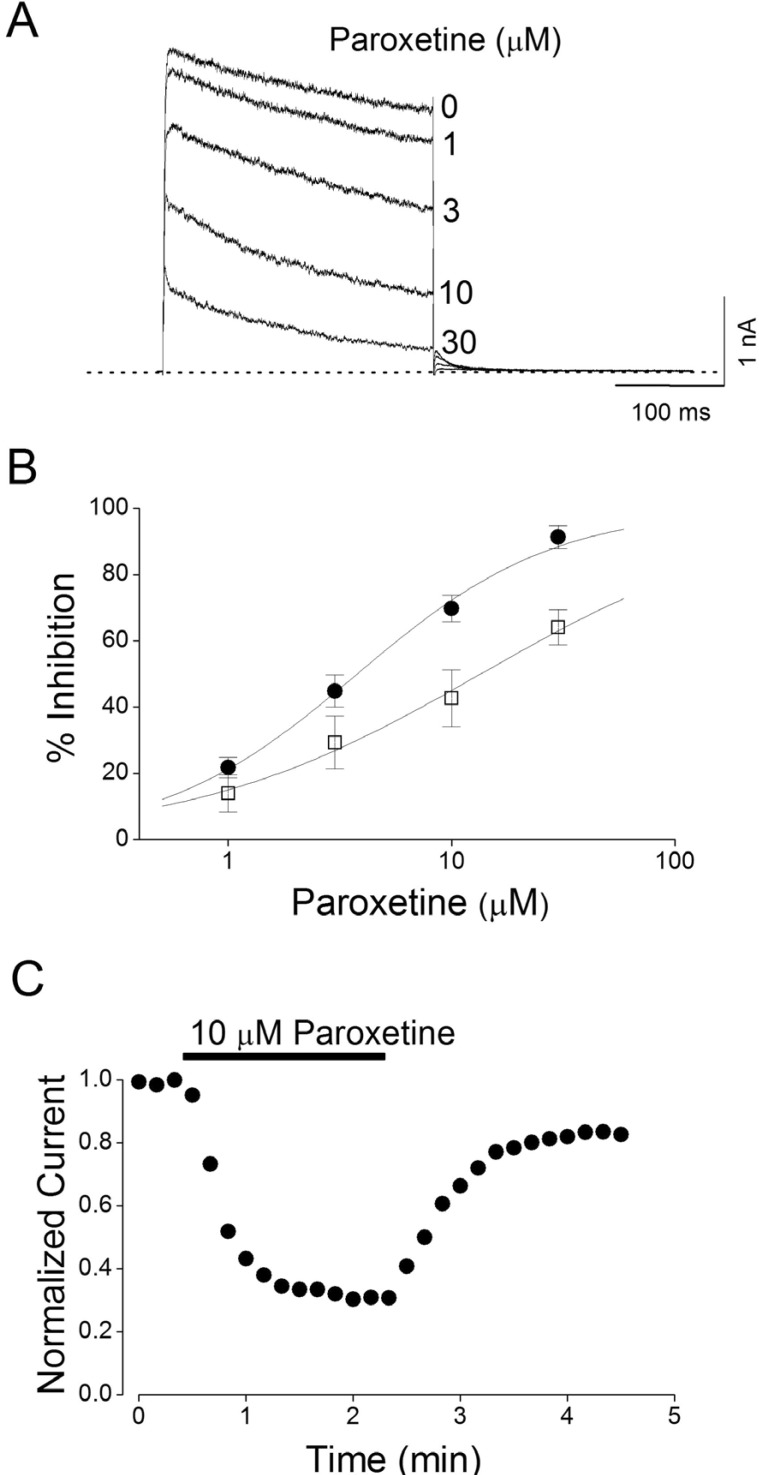
- Paroxetine, a selective serotonin reuptake inhibitor (SSRI), has been reported to have an effect on several ion channels including human ether-a-go-go-related gene in a SSRI-independent manner. These results suggest that paroxetine may cause side effects on cardiac system. In this study, we investigated the effect of paroxetine on Kv1.5, which is one of cardiac ion channels. The action of paroxetine on the cloned neuronal rat Kv1.5 channels stably expressed in Chinese hamster ovary cells was investigated using the whole-cell patch-clamp technique. Paroxetine reduced Kv1.5 whole-cell currents in a reversible concentration-dependent manner, with an IC50 value and a Hill coefficient of 4.11 microM and 0.98, respectively. Paroxetine accelerated the decay rate of inactivation of Kv1.5 currents without modifying the kinetics of current activation. The inhibition increased steeply between -30 and 0 mV, which corresponded with the voltage range for channel opening. In the voltage range positive to 0 mV, inhibition displayed a weak voltage dependence, consistent with an electrical distance delta of 0.32. The binding (k(+1)) and unbinding (k(-1)) rate constants for paroxetine-induced block of Kv1.5 were 4.9 microM(-1)s(-1) and 16.1 s-1, respectively. The theoretical K(D) value derived by k(-1)/k(+1) yielded 3.3 microM. Paroxetine slowed the deactivation time course, resulting in a tail crossover phenomenon when the tail currents, recorded in the presence and absence of paroxetine, were superimposed. Inhibition of Kv1.5 by paroxetine was use-dependent. The present results suggest that paroxetine acts on Kv1.5 currents as an open-channel blocker.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Facilitation of serotonin-induced contraction of rat mesenteric artery by ketamine
Sang Woong Park, Hyun Ju Noh, Jung Min Kim, Bokyung Kim, Sung-Il Cho, Yoon Soo Kim, Nam Sik Woo, Sung Hun Kim, Young Min Bae
Korean J Physiol Pharmacol. 2016;20(6):605-611. doi: 10.4196/kjpp.2016.20.6.605.Nortriptyline, a tricyclic antidepressant, inhibits voltage-dependent K+ channels in coronary arterial smooth muscle cells
Sung Eun Shin, Hongliang Li, Han Sol Kim, Hye Won Kim, Mi Seon Seo, Kwon-Soo Ha, Eun-Taek Han, Seok-Ho Hong, Amy L. Firth, Il-Whan Choi, Young Min Bae, Won Sun Park
Korean J Physiol Pharmacol. 2017;21(2):225-232. doi: 10.4196/kjpp.2017.21.2.225.The antidiabetic drug rosiglitazone blocks Kv1.5 potassium channels in an open state
Hyang Mi Lee, Sang June Hahn, Bok Hee Choi
Korean J Physiol Pharmacol. 2022;26(2):135-144. doi: 10.4196/kjpp.2022.26.2.135.
Reference
-
1. Henry JA, Alexander CA, Sener EK. Relative mortality from overdose of antidepressants. BMJ. 1995; 310:221–224. PMID: 7866123.
Article2. Henry JA. Epidemiology and relative toxicity of antidepressant drugs in overdose. Drug Saf. 1997; 16:374–390. PMID: 9241492.
Article3. Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SH. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet. 2000; 355:1048–1052. PMID: 10744090.
Article4. Scherer D, von Löwenstern K, Zitron E, Scholz EP, Bloehs R, Kathöfer S, Thomas D, Bauer A, Katus HA, Karle CA, Kiesecker C. Inhibition of cardiac hERG potassium channels by tetracyclic antidepressant mianserin. Naunyn Schmiedebergs Arch Pharmacol. 2008; 378:73–83. PMID: 18458880.
Article5. Hyttel J. Citalopram--pharmacological profile of a specific serotonin uptake inhibitor with antidepressant activity. Prog Neuropsychopharmacol Biol Psychiatry. 1982; 6:277–295. PMID: 6128769.6. Wong DT, Bymaster FP, Engleman EA. Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 1995; 57:411–441. PMID: 7623609.
Article7. Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. J Affect Disord. 2000; 58:19–36. PMID: 10760555.
Article8. Montgomery SA. A meta-analysis of the efficacy and tolerability of paroxetine versus tricyclic antidepressants in the treatment of major depression. Int Clin Psychopharmacol. 2001; 16:169–178. PMID: 11354239.
Article9. Wang GK, Mitchell J, Wang SY. Block of persistent late Na+ currents by antidepressant sertraline and paroxetine. J Membr Biol. 2008; 222:79–90. PMID: 18418539.10. Dick IE, Brochu RM, Purohit Y, Kaczorowski GJ, Martin WJ, Priest BT. Sodium channel blockade may contribute to the analgesic efficacy of antidepressants. J Pain. 2007; 8:315–324. PMID: 17175203.
Article11. Huang CJ, Harootunian A, Maher MP, Quan C, Raj CD, McCormack K, Numann R, Negulescu PA, González JE. Characterization of voltage-gated sodium-channel blockers by electrical stimulation and fluorescence detection of membrane potential. Nat Biotechnol. 2006; 24:439–446. PMID: 16550174.
Article12. Kobayashi T, Washiyama K, Ikeda K. Inhibition of G protein-activated inwardly rectifying K+ channels by the antidepressant paroxetine. J Pharmacol Sci. 2006; 102:278–287. PMID: 17072103.13. Thümmler S, Duprat F, Lazdunski M. Antipsychotics inhibit TREK but not TRAAK channels. Biochem Biophys Res Commun. 2007; 354:284–289. PMID: 17222806.
Article14. Lee SH, Sung MJ, Lee HM, Chu D, Hahn SJ, Jo SH, Choe H, Choi BH. Blockade of HERG human K+ channels by the antidepressant drug paroxetine. Biol Pharm Bull. 2014; 37:1495–1504. PMID: 25177033.
Article15. Wang Z, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 1993; 73:1061–1076. PMID: 8222078.16. Swanson R, Marshall J, Smith JS, Williams JB, Boyle MB, Folander K, Luneau CJ, Antanavage J, Oliva C, Buhrow SA, Bennet C, Stein RB, Kaczmarek LK. Cloning and expression of cDNA and genomic clones encoding three delayed rectifier potassium channels in rat brain. Neuron. 1990; 4:929–939. PMID: 2361015.
Article17. Colatsky TJ, Follmer CH, Starmer CF. Channel specificity in antiarrhythmic drug action. Mechanism of potassium channel block and its role in suppressing and aggravating cardiac arrhythmias. Circulation. 1990; 82:2235–2242. PMID: 2242545.
Article18. Li GR, Feng J, Wang Z, Fermini B, Nattel S. Adrenergic modulation of ultrarapid delayed rectifier K+ current in human atrial myocytes. Circ Res. 1996; 78:903–915. PMID: 8620611.19. Cobbe SM. Incidence and risks associated with atrial fibrillation. Pacing Clin Electrophysiol. 1994; 17:1005–1010. PMID: 7518586.
Article20. Choi BH, Choi JS, Jeong SW, Hahn SJ, Yoon SH, Jo YH, Kim MS. Direct block by bisindolylmaleimide of rat Kv1.5 expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 2000; 293:634–640. PMID: 10773038.21. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981; 391:85–100. PMID: 6270629.
Article22. Snyders DJ, Yeola SW. Determinants of antiarrhythmic drug action. Electrostatic and hydrophobic components of block of the human cardiac hKv1.5 channel. Circ Res. 1995; 77:575–583. PMID: 7641327.23. Woodhull AM. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973; 61:687–708. PMID: 4541078.
Article24. Snyders DJ, Tamkun MM, Bennett PB. A rapidly activating and slowly inactivating potassium channel cloned from human heart. Functional analysis after stable mammalian cell culture expression. J Gen Physiol. 1993; 101:513–543. PMID: 8505626.
Article25. Valenzuela C, Delpón E, Franqueza L, Gay P, Pérez O, Tamargo J, Snyders DJ. Class III antiarrhythmic effects of zatebradine. Time-, state-, use-, and voltage-dependent block of hKv1.5 channels. Circulation. 1996; 94:562–570. PMID: 8759103.26. Delpón E, Valenzuela C, Gay P, Franqueza L, Snyders DJ, Tamargo J. Block of human cardiac Kv1.5 channels by loratadine: voltage-, time- and use-dependent block at concentrations above therapeutic levels. Cardiovasc Res. 1997; 35:341–350. PMID: 9349397.
Article27. Franqueza L, Valenzuela C, Delpón E, Longobardo M, Caballero R, Tamargo J. Effects of propafenone and 5-hydroxy-propafenone on hKv1.5 channels. Br J Pharmacol. 1998; 125:969–978. PMID: 9846634.
Article28. Choi JS, Hahn SJ, Rhie DJ, Yoon SH, Jo YH, Kim MS. Mechanism of fluoxetine block of cloned voltage-activated potassium channel Kv1.3. J Pharmacol Exp Ther. 1999; 291:1–6. PMID: 10490879.29. Vasskog T, Berger U, Samuelsen PJ, Kallenborn R, Jensen E. Selective serotonin reuptake inhibitors in sewage influents and effluents from Tromsø, Norway. J Chromatogr A. 2006; 1115:187–195. PMID: 16574138.
Article30. Snyders J, Knoth KM, Roberds SL, Tamkun MM. Time-, voltage-, and state-dependent block by quinidine of a cloned human cardiac potassium channel. Mol Pharmacol. 1992; 41:322–330. PMID: 1538710.31. Lee HM, Hahn SJ, Choi BH. Open channel block of Kv1.5 currents by citalopram. Acta Pharmacol Sin. 2010; 31:429–435. PMID: 20228830.
Article32. Baldessarini R. Drugs and the treatment of psychiatric disorders: depression and anxiety disorders. In : Hardman JG, Limbird LE, Gilman AG, editors. The pharmacological basis of therapeutics. New York: McGraw-Hill;2001. p. 447–484.33. Aréchiga IA, Barrio-Echavarria GF, Rodríguez-Menchaca AA, Moreno-Galindo EG, Decher N, Tristani-Firouzi M, Sánchez-Chapula JA, Navarro-Polanco RA. Kv1.5 open channel block by the antiarrhythmic drug disopyramide: molecular determinants of block. J Pharmacol Sci. 2008; 108:49–55. PMID: 18818480.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The antidiabetic drug rosiglitazone blocks Kv1.5 potassium channels in an open state
- Antidepressant-Induced Somnabulism: A Case of Mirtazapin
- Combined Therapy of Paroxetine and Tricyclic Antidepressant in Depression of Schizophrenic Patients
- Blockade of Kv1.5 channels by the antidepressant drug sertraline
- Selection of an Antidepressant Based on the Genotypes of Cytochrome P450 Enzymes Genes in a Patient with Major Depressive Disorder