Child Kidney Dis.
2024 Jun;28(2):51-58. 10.3339/ckd.24.006.
How to delay the progression of chronic kidney disease: focusing on medications
- Affiliations
-
- 1Department of Pediatrics, Chungnam National University Sejong Hospital, Sejong, Republic of Korea
- KMID: 2557070
- DOI: http://doi.org/10.3339/ckd.24.006
Abstract
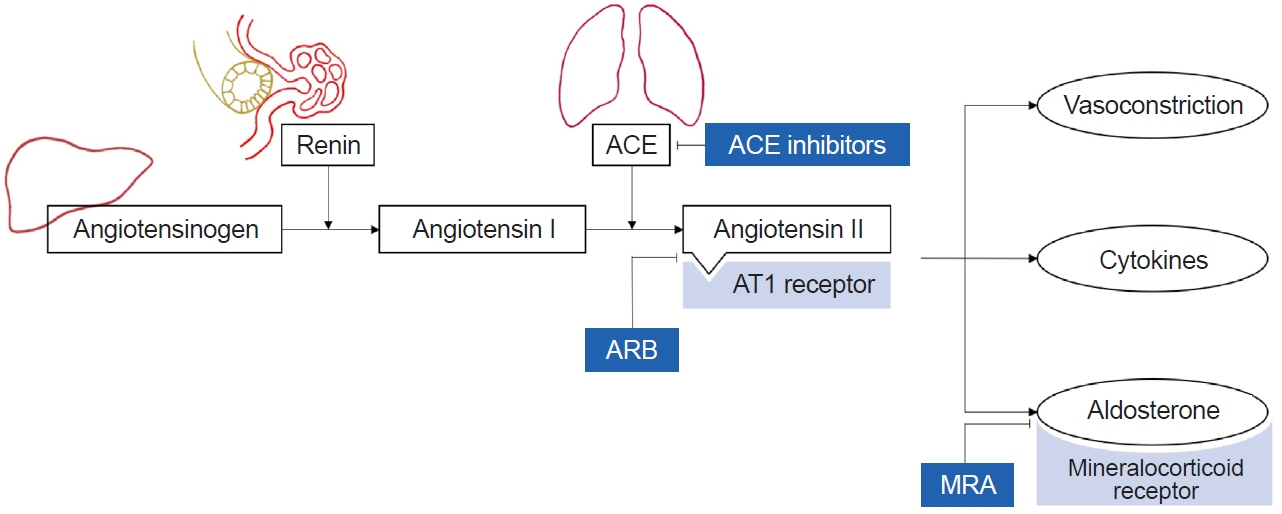
- Patients with chronic kidney disease (CKD) bear a significant financial burden and face numerous complications and higher mortality rates. The progression of CKD is associated with glomerular injury caused by glomerular hyperfiltration and oxidative stress. Factors such as uncontrolled hypertension, elevated urine protein levels, anemia, and underlying glomerular disease, contribute to CKD progression. In addition to conservative treatment, several medications are available to combat the progression of CKD to end-stage kidney disease. Renin-angiotensin-aldosterone system blockers could slow the progression of CKD by reducing glomerular hyperfiltration, lowering blood pressure, and decreasing inflammation. Mineralocorticoid receptor antagonists inhibit the mineralocorticoid receptor signaling pathway, thereby attenuating inflammation and fibrosis. Sodium-glucose cotransporter 2 inhibitors exhibit protective effects on the kidneys and against cardiovascular events. Tolvaptan, a selective vasopressin V2-receptor antagonist, decelerates the rate of increase in total kidney volume and deterioration of kidney function in patients with rapidly progressive autosomal dominant polycystic kidney disease. The protective effects of AST-120 remain controversial. Due to a lack of evidence regarding the efficacy and safety of these medications in children, it is imperative to weigh the benefits and adverse effects carefully. Further research is essential to establish the efficacy and safety profiles in pediatric populations.
Keyword
Figure
Reference
-
References
1. Chapter 2: Definition, identification, and prediction of CKD progression. Kidney Int Suppl (2011). 2013; 3:63–72.2. Warady BA, Abraham AG, Schwartz GJ, Wong CS, Munoz A, Betoko A, et al. Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: the Chronic Kidney Disease in Children (CKiD) Cohort. Am J Kidney Dis. 2015; 65:878–88.3. Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011). 2013; 3:19–62.4. Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012; 27:363–73.
Article5. Furth SL, Abraham AG, Jerry-Fluker J, Schwartz GJ, Benfield M, Kaskel F, et al. Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol. 2011; 6:2132–40.
Article6. Staples AO, Greenbaum LA, Smith JM, Gipson DS, Filler G, Warady BA, et al. Association between clinical risk factors and progression of chronic kidney disease in children. Clin J Am Soc Nephrol. 2010; 5:2172–9.
Article7. Kamath N, Iyengar A, George N, Luyckx VA. Risk factors and rate of progression of CKD in children. Kidney Int Rep. 2019; 4:1472–7.
Article8. Park PG, Kang HG, Park E, Ahn YH, Choi HJ, Han KH, et al. Baseline characteristics of participants enrolled in the KoreaN cohort study for Outcomes in patients With Pediatric Chronic Kidney Disease (KNOW-Ped CKD). Pediatr Nephrol. 2022; 37:3177–87.
Article9. Okuda Y, Soohoo M, Ishikura K, Tang Y, Obi Y, Laster M, et al. Primary causes of kidney disease and mortality in dialysis-dependent children. Pediatr Nephrol. 2020; 35:851–60.
Article10. Becherucci F, Roperto RM, Materassi M, Romagnani P. Chronic kidney disease in children. Clin Kidney J. 2016; 9:583–91.
Article11. Chapter 3: Management of progression and complications of CKD. Kidney Int Suppl (2011). 2013; 3:73–90.12. Lopez-Novoa JM, Martinez-Salgado C, Rodriguez-Pena AB, Lopez-Hernandez FJ. Common pathophysiological mechanisms of chronic kidney disease: therapeutic perspectives. Pharmacol Ther. 2010; 128:61–81.
Article13. Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982; 307:652–9.14. Denic A, Mathew J, Lerman LO, Lieske JC, Larson JJ, Alexander MP, et al. Single-nephron glomerular filtration rate in healthy adults. N Engl J Med. 2017; 376:2349–57.
Article15. Daenen K, Andries A, Mekahli D, Van Schepdael A, Jouret F, Bammens B. Oxidative stress in chronic kidney disease. Pediatr Nephrol. 2019; 34:975–91.
Article16. Cerqueira DC, Soares CM, Silva VR, Magalhaes JO, Barcelos IP, Duarte MG, et al. A predictive model of progression of CKD to ESRD in a predialysis pediatric interdisciplinary program. Clin J Am Soc Nephrol. 2014; 9:728–35.
Article17. Fathallah-Shaykh SA, Flynn JT, Pierce CB, Abraham AG, Blydt-Hansen TD, Massengill SF, et al. Progression of pediatric CKD of nonglomerular origin in the CKiD cohort. Clin J Am Soc Nephrol. 2015; 10:571–7.
Article18. Furth SL, Pierce C, Hui WF, White CA, Wong CS, Schaefer F, et al. Estimating time to ESRD in children with CKD. Am J Kidney Dis. 2018; 71:783–92.19. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021; 99(3S):S1–87.20. Stevenson JK, Campbell ZC, Webster AC, Chow CK, Tong A, Craig JC, et al. eHealth interventions for people with chronic kidney disease. Cochrane Database Syst Rev. 2019; 8:CD012379.
Article21. Cirillo L, De Chiara L, Innocenti S, Errichiello C, Romagnani P, Becherucci F. Chronic kidney disease in children: an update. Clin Kidney J. 2023; 16:1600–11.
Article22. KDOQI Work Group. KDOQI clinical practice guideline for nutrition in children with CKD: 2008 update: executive summary. Am J Kidney Dis. 2009; 53(3 Suppl 2):S11–104.23. Robles NR, Cerezo I, Hernandez-Gallego R. Renin-angiotensin system blocking drugs. J Cardiovasc Pharmacol Ther. 2014; 19:14–33.
Article24. Remuzzi A, Puntorieri S, Battaglia C, Bertani T, Remuzzi G. Angiotensin converting enzyme inhibition ameliorates glomerular filtration of macromolecules and water and lessens glomerular injury in the rat. J Clin Invest. 1990; 85:541–9.
Article25. Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005; 68(Suppl 99):S57–65.
Article26. Dandona P, Dhindsa S, Ghanim H, Chaudhuri A. Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens. 2007; 21:20–7.
Article27. Drawz PE, Beddhu S, Bignall ONR 2nd, Cohen JB, Flynn JT, Ku E, et al. KDOQI US commentary on the 2021 KDIGO clinical practice guideline for the management of blood pressure in CKD. Am J Kidney Dis. 2022; 79:311–27.
Article28. ESCAPE Trial Group, Wuhl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009; 361:1639–50.
Article29. Abraham AG, Betoko A, Fadrowski JJ, Pierce C, Furth SL, Warady BA, et al. Renin-angiotensin II-aldosterone system blockers and time to renal replacement therapy in children with CKD. Pediatr Nephrol. 2017; 32:643–9.
Article30. Herman LL, Padala SA, Ahmed I, Bashir. K. Angiotensin-converting enzyme inhibitors (ACEI). In: StatPearls [Internet]. StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK431051/.31. Rodgers JE, Patterson JH. Angiotensin II-receptor blockers: clinical relevance and therapeutic role. Am J Health Syst Pharm. 2001; 58:671–83.
Article32. Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ. 2013; 346:f360.
Article33. Azizi M, Menard J. Combined blockade of the renin-angiotensin system with angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists. Circulation. 2004; 109:2492–9.
Article34. Stotter BR, Ferguson MA. Should ACE inhibitors and ARBs be used in combination in children? Pediatr Nephrol. 2019; 34:1521–32.
Article35. Georgianos PI, Agarwal R. Mineralocorticoid receptor antagonism in chronic kidney disease. Kidney Int Rep. 2021; 6:2281–91.
Article36. Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, Wada T, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021; 42:152–61.
Article37. Chung EY, Ruospo M, Natale P, Bolignano D, Navaneethan SD, Palmer SC, et al. Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2020; 10:CD007004.
Article38. Hasegawa T, Nishiwaki H, Ota E, Levack WM, Noma H. Aldosterone antagonists for people with chronic kidney disease requiring dialysis. Cochrane Database Syst Rev. 2021; 2:CD013109.
Article39. Thomsen RW, Nicolaisen SK, Hasvold P, Sanchez RG, Pedersen L, Adelborg K, et al. Elevated potassium levels in patients with chronic kidney disease: occurrence, risk factors and clinical outcomes: a Danish population-based cohort study. Nephrol Dial Transplant. 2018; 33:1610–20.40. Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020; 383:2219–29.
Article41. Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021; 385:2252–63.
Article42. Bayer. A study to learn more about how well the study treatment finerenone works, how safe it is, how it moves into, through, and out of the body, and the effects it has on the body when taken with an ace inhibitor or angiotensin receptor blocker in children with chronic kidney disease and proteinuria (FIONA). ClinicalTrials.gov identifier: NCT05196035 [cited May 11, 2024]. Available from: https://clinicaltrials.gov/study/NCT05196035.43. Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022; 43:474–84.
Article44. Chao EC, Henry RR. SGLT2 inhibition: a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010; 9:551–9.
Article45. Verma S, McMurray JJ. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018; 61:2108–17.
Article46. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–28.
Article47. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016; 375:323–34.
Article48. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019; 380:2295–306.
Article49. Heerspink HJ, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020; 383:1436–46.
Article50. Wheeler DC, Toto RD, Stefansson BV, Jongs N, Chertow GM, Greene T, et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021; 100:215–24.
Article51. Wheeler DC, Jongs N, Stefansson BV, Chertow GM, Greene T, Hou FF, et al. Safety and efficacy of dapagliflozin in patients with focal segmental glomerulosclerosis: a prespecified analysis of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial. Nephrol Dial Transplant. 2022; 37:1647–56.
Article52. Liu J, Cui J, Fang X, Chen J, Yan W, Shen Q, et al. Efficacy and safety of dapagliflozin in children with inherited proteinuric kidney disease: a pilot study. Kidney Int Rep. 2021; 7:638–41.
Article53. Scheen AJ. An update on the safety of SGLT2 inhibitors. Expert Opin Drug Saf. 2019; 18:295–311.
Article54. Qiu M, Ding LL, Zhang M, Zhou HR. Safety of four SGLT2 inhibitors in three chronic diseases: a meta-analysis of large randomized trials of SGLT2 inhibitors. Diab Vasc Dis Res. 2021; 18:14791641211011016.
Article55. Heerspink HJ, Cherney DZ. Clinical implications of an acute dip in eGFR after SGLT2 inhibitor initiation. Clin J Am Soc Nephrol. 2021; 16:1278–80.
Article56. Gross O. Phase 3 clinical trial with dapagliflozin in chronic kidney disease in adolescents and young adult patients (DOUBLE_PROTECT). ClinicalTrials.gov identifier: NCT05944016 [cited May 11, 2024]. Available from: https://clinicaltrials.gov/study/NCT05944016.57. Hannover Medical School. Study of empagliflozin in patients with autosomal dominant polycystic kidney disease (EMPA-PKD). ClinicalTrials.gov identifier: NCT06391450 [cited May 11, 2024]. Available from: https://clinicaltrials.gov/study/NCT06391450.58. Bayer. A study to learn how well the treatment combination of finerenone and empagliflozin works and how safe it is compared to each treatment alone in adult participants with long-term kidney disease (chronic kidney disease) and type 2 diabetes (CONFIDENCE). ClinicalTrials.gov identifier: NCT05254002 [cited May 11, 2024]. Available from: https://clinicaltrials.gov/study/NCT05254002.59. Dell KM. The spectrum of polycystic kidney disease in children. Adv Chronic Kidney Dis. 2011; 18:339–47.
Article60. Chapman AB, Devuyst O, Eckardt KU, Gansevoort RT, Harris T, Horie S, et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2015; 88:17–27.
Article61. Miyazaki T, Fujiki H, Yamamura Y, Nakamura S, Mori T. Tolvaptan, an orally active vasopressin V(2)-receptor antagonist: pharmacology and clinical trials. Cardiovasc Drug Rev. 2007; 25:1–13.
Article62. Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012; 367:2407–18.
Article63. Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017; 377:1930–42.
Article64. Gansevoort RT, Arici M, Benzing T, Birn H, Capasso G, Covic A, et al. Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant. 2016; 31:337–48.
Article65. Mekahli D, Guay-Woodford LM, Cadnapaphornchai MA, Greenbaum LA, Litwin M, Seeman T, et al. Tolvaptan for children and adolescents with autosomal dominant polycystic kidney disease: randomized controlled trial. Clin J Am Soc Nephrol. 2023; 18:36–46.
Article66. Akizawa T, Asano Y, Morita S, Wakita T, Onishi Y, Fukuhara S, et al. Effect of a carbonaceous oral adsorbent on the progression of CKD: a multicenter, randomized, controlled trial. Am J Kidney Dis. 2009; 54:459–67.
Article67. Asai M, Kumakura S, Kikuchi M. Review of the efficacy of AST-120 (KREMEZIN®) on renal function in chronic kidney disease patients. Ren Fail. 2019; 41:47–56.
Article68. Cha RH, Kang SW, Park CW, Cha DR, Na KY, Kim SG, et al. A randomized, controlled trial of oral intestinal sorbent AST-120 on renal function deterioration in patients with advanced renal dysfunction. Clin J Am Soc Nephrol. 2016; 11:559–67.
Article69. Chen YC, Wu MY, Hu PJ, Chen TT, Shen WC, Chang WC, et al. Effects and safety of an oral adsorbent on chronic kidney disease progression: a systematic review and meta-analysis. J Clin Med. 2019; 8:1718.
Article70. Schulman G, Agarwal R, Acharya M, Berl T, Blumenthal S, Kopyt N. A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study of AST-120 (Kremezin) in patients with moderate to severe CKD. Am J Kidney Dis. 2006; 47:565–77.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Risk Factors for the Progression of Chronic Kidney Disease in Children
- Clinical Nutrition Therapy of Diabetic Nephropathy
- Slowing the Progression of Chronic Kidney Disease in Children and Adolescents
- Very low protein plus ketoacid analogs of essential aminoacids do not confirm superiority of a low protein diet to retard chronic kidney disease progression
- The association between acute kidney injury in renal infarction and progression to chronic kidney disease