Kosin Med J.
2024 Mar;39(1):5-17. 10.7180/kmj.24.104.
Clinical challenges and advancements in diagnosing Staphylococcus aureus-associated musculoskeletal infections
- Affiliations
-
- 1Department of Orthopaedics and Rehabilitation, Yale School of Medicine, New Haven, CT, USA
- KMID: 2556793
- DOI: http://doi.org/10.7180/kmj.24.104
Abstract
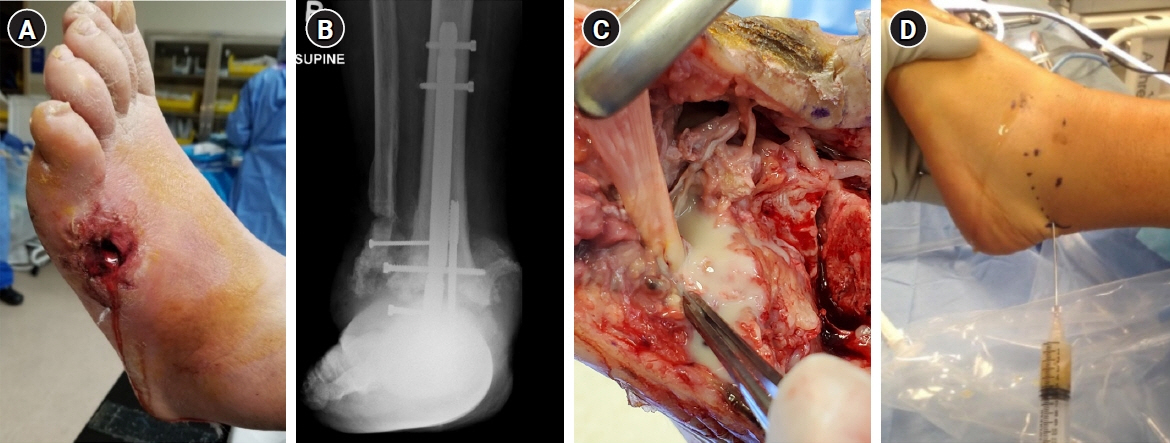
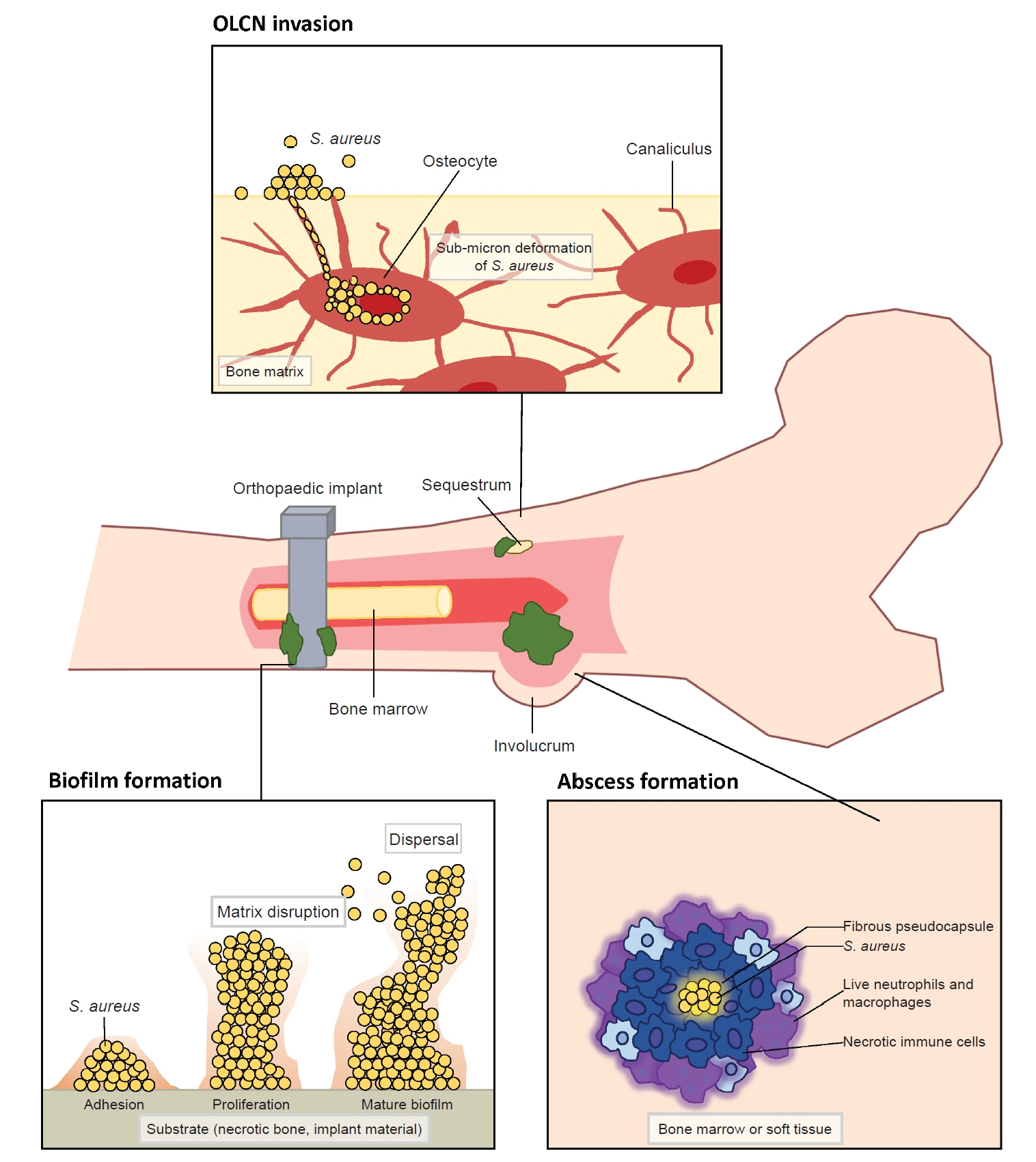
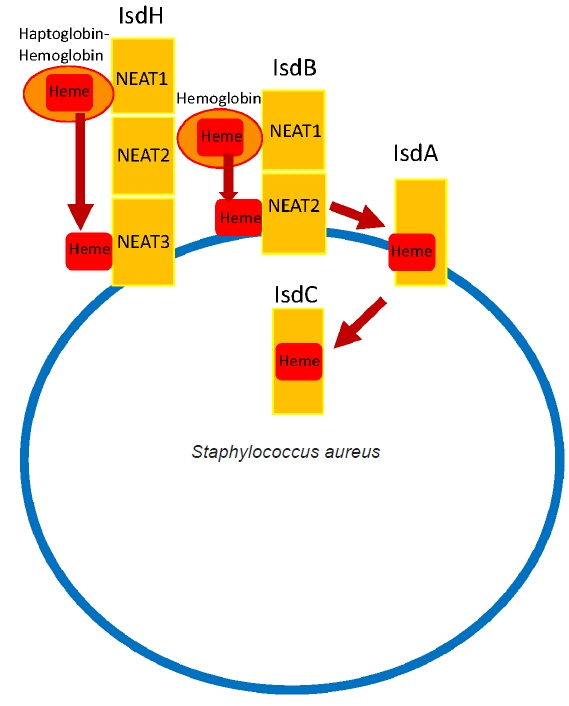
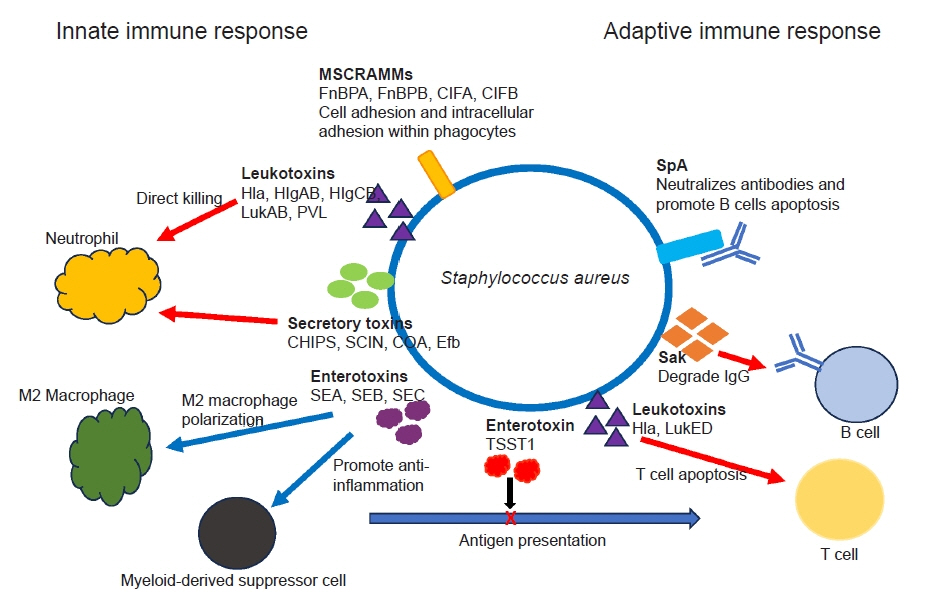
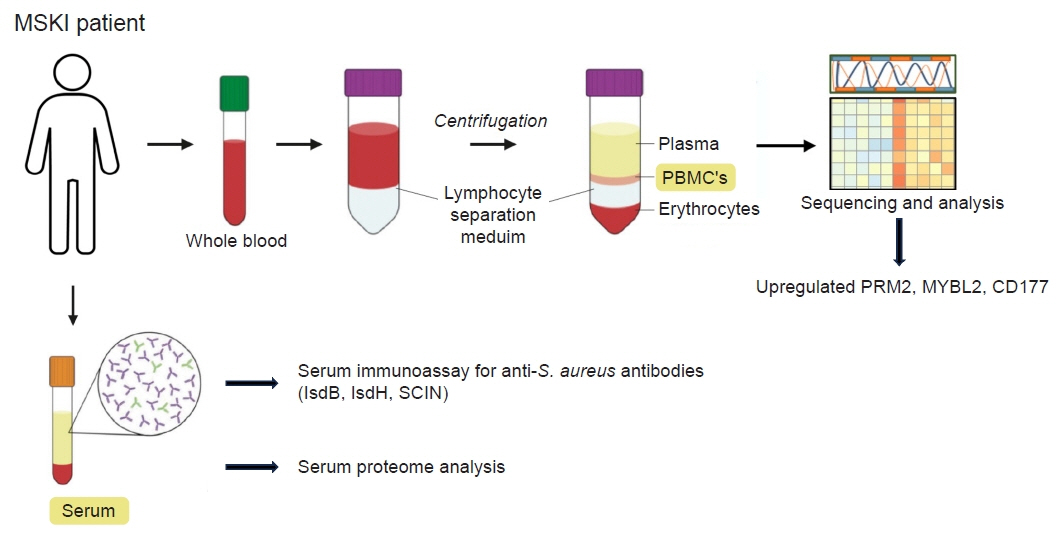
- Musculoskeletal infections (MSKI) present a significant health challenge, with a rising incidence linked to the aging population and advancements in orthopedic surgical care. Staphylococcus aureus is the most prevalent pathogen associated with orthopedic infections. The conventional culture method for identification of pathogen frequently lacks accuracy and is challenged by false-positive or false-negative results. Inflammatory markers such as the erythrocyte sedimentation rate and C-reactive protein are not site-specific or accurate, as they can be confounded by other medical conditions. Identifying the dominant pathogen and monitoring treatment response following surgical debridement and antibiotics therapy continues to pose challenges. Understanding the pathogenesis of MSKI is crucial for the development of innovative diagnostics and alternative therapeutics. S. aureus immune evasion stands out as a key component of the pathogenic mechanism, complicating clinical decisions. Other unique mechanisms such as biofilm and abscess formation, as well as osteocyte-lacuno canalicular network invasion, underscore the need for aggressive debridement and the complete removal of infected implants and bone tissues. Ongoing efforts focus on exploring and developing innovative diagnostics, such as serum immunoassays, next-generation sequencing of infected tissue, transcriptomics of peripheral blood mononuclear cells, and serum proteomics. These endeavors offer promising avenues for improved diagnostics, medical management, and innovative therapeutics for MSKI.
Figure
Reference
-
References
1. Nishitani K, Beck CA, Rosenberg AF, Kates SL, Schwarz EM, Daiss JL. A diagnostic serum antibody test for patients with Staphylococcus aureus osteomyelitis. Clin Orthop Relat Res. 2015; 473:2735–49.2. Nishitani K, Sutipornpalangkul W, de Mesy Bentley KL, Varrone JJ, Bello-Irizarry SN, Ito H, et al. Quantifying the natural history of biofilm formation in vivo during the establishment of chronic implant-associated Staphylococcus aureus osteomyelitis in mice to identify critical pathogen and host factors. J Orthop Res. 2015; 33:1311–9.3. Masters EA, Trombetta RP, de Mesy Bentley KL, Boyce BF, Gill AL, Gill SR, et al. Evolving concepts in bone infection: redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. Bone Res. 2019; 7:20.4. Masters EA, Ricciardi BF, Bentley KL, Moriarty TF, Schwarz EM, Muthukrishnan G. Skeletal infections: microbial pathogenesis, immunity and clinical management. Nat Rev Microbiol. 2022; 20:385–400.5. Longo M, Pennington Z, Gelfand Y, De la Garza Ramos R, Echt M, Ahmed AK, et al. Readmission after spinal epidural abscess management in urban populations: a bi-institutional study. J Neurosurg Spine. 2019; 32:465–72.6. Beam E, Osmon D. Prosthetic joint infection update. Infect Dis Clin North Am. 2018; 32:843–59.7. Oh I, Muthukrishnan G, Ninomiya MJ, Brodell JD Jr, Smith BL, Lee CC, et al. Tracking anti-Staphylococcus aureus antibodies produced in vivo and ex vivo during foot salvage therapy for diabetic foot infections reveals prognostic insights and evidence of diversified humoral immunity. Infect Immun. 2018; 86:e00629–18.8. Lipof JS, Jones CM, Daiss J, Oh I. Comparative study of culture, next-generation sequencing, and immunoassay for identification of pathogen in diabetic foot ulcer. J Orthop Res. 2021; 39:2638–45.9. Sulovari A, Ninomiya MJ, Beck CA, Ricciardi BF, Ketonis C, Mesfin A, et al. Clinical utilization of species-specific immunoassays for identification of Staphylococcus aureus and Streptococcus agalactiae in orthopedic infections. J Orthop Res. 2021; 39:2141–50.10. Chaussade H, Uckay I, Vuagnat A, Druon J, Gras G, Rosset P, et al. Antibiotic therapy duration for prosthetic joint infections treated by debridement and implant retention (DAIR): similar long-term remission for 6 weeks as compared to 12 weeks. Int J Infect Dis. 2017; 63:37–42.11. Li HK, Rombach I, Zambellas R, Walker AS, McNally MA, Atkins BL, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019; 380:425–36.12. Scarborough M, Li HK, Rombach I, Zambellas R, Walker AS, McNally M, et al. Oral versus intravenous antibiotics for bone and joint infections: the OVIVA non-inferiority RCT. Health Technol Assess. 2019; 23:1–92.13. Blumberg G, Long B, Koyfman A. Clinical mimics: an emergency medicine-focused review of cellulitis mimics. J Emerg Med. 2017; 53:475–84.14. Uy JP, Nuwayhid N, Saadeh C. Unusual presentations of gout: tips for accurate diagnosis. Postgrad Med. 1996; 100:253–68.15. Lipsky BA, Senneville E, Abbas ZG, Aragon-Sanchez J, Diggle M, Embil JM, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020; 36 Suppl 1:e3280.16. Lu K, Zhang J, Cheng J, Liu H, Yang C, Yin L, et al. Incidence and risk factors for surgical site infection after open reduction and internal fixation of intra-articular fractures of distal femur: a multicentre study. Int Wound J. 2019; 16:473–8.17. McDonald LS, Bavaro MF, Hofmeister EP, Kroonen LT. Hand infections. J Hand Surg Am. 2011; 36:1403–12.18. Park KH, Kim DY, Lee YM, Lee MS, Kang KC, Lee JH, et al. Selection of an appropriate empiric antibiotic regimen in hematogenous vertebral osteomyelitis. PLoS One. 2019; 14:e0211888.19. Ricciardi BF, Muthukrishnan G, Masters EA, Kaplan N, Daiss JL, Schwarz EM. New developments and future challenges in prevention, diagnosis, and treatment of prosthetic joint infection. J Orthop Res. 2020; 38:1423–35.20. Holtfreter S, Kolata J, Broker BM. Towards the immune proteome of Staphylococcus aureus: the anti-S. aureus antibody response. Int J Med Microbiol. 2010; 300:176–92.21. Lebon A, Labout JA, Verbrugh HA, Jaddoe VW, Hofman A, van Wamel W, et al. Dynamics and determinants of Staphylococcus aureus carriage in infancy: the Generation R Study. J Clin Microbiol. 2008; 46:3517–21.22. Howden BP, Giulieri SG, Wong Fok Lung T, Baines SL, Sharkey LK, Lee JY, et al. Staphylococcus aureus host interactions and adaptation. Nat Rev Microbiol. 2023; 21:380–95.23. Jenkins A, Diep BA, Mai TT, Vo NH, Warrener P, Suzich J, et al. Differential expression and roles of Staphylococcus aureus virulence determinants during colonization and disease. mBio. 2015; 6:e02272–14.24. de Mesy Bentley KL, MacDonald A, Schwarz EM, Oh I. Chronic osteomyelitis with Staphylococcus aureus deformation in submicron canaliculi of osteocytes: a case report. JBJS Case Connect. 2018; 8:e8.25. Guo H, Tong Y, Cheng J, Abbas Z, Li Z, Wang J, et al. Biofilm and small colony variants: an update on Staphylococcus aureus strategies toward drug resistance. Int J Mol Sci. 2022; 23:1241.26. Muthukrishnan G, Masters EA, Daiss JL, Schwarz EM. Mechanisms of Immune evasion and bone tissue colonization that make Staphylococcus aureus the primary pathogen in osteomyelitis. Curr Osteoporos Rep. 2019; 17:395–404.27. Hao SP, Masters EA, Ninomiya MJ, Beck CA, Schwarz EM, Daiss JL, et al. Species-specific immunoassay aids identification of pathogen and tracks infectivity in foot infection. Foot Ankle Int. 2021; 42:363–72.28. Bennett MR, Dong J, Bombardi RG, Soto C, Parrington HM, Nargi RS, et al. Human VH1-69 gene-encoded human monoclonal antibodies against Staphylococcus aureus IsdB use at least three distinct modes of binding to inhibit bacterial growth and pathogenesis. mBio. 2019; 10:e02473–19.29. Pishchany G, Sheldon JR, Dickson CF, Alam MT, Read TD, Gell DA, et al. IsdB-dependent hemoglobin binding is required for acquisition of heme by Staphylococcus aureus. J Infect Dis. 2014; 209:1764–72.30. Torres VJ, Pishchany G, Humayun M, Schneewind O, Skaar EP. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J Bacteriol. 2006; 188:8421–9.31. Tsai CM, Caldera JR, Hajam IA, Chiang AW, Tsai CH, Li H, et al. Non-protective immune imprint underlies failure of Staphylococcus aureus IsdB vaccine. Cell Host Microbe. 2022; 30:1163–72.32. McNeely TB, Shah NA, Fridman A, Joshi A, Hartzel JS, Keshari RS, et al. Mortality among recipients of the Merck V710 Staphylococcus aureus vaccine after postoperative S. aureus infections: an analysis of possible contributing host factors. Hum Vaccin Immunother. 2014; 10:3513–6.33. Nishitani K, Ishikawa M, Morita Y, Yokogawa N, Xie C, de Mesy Bentley KL, et al. IsdB antibody-mediated sepsis following S. aureus surgical site infection. JCI Insight. 2020; 5:e141164.34. Rigby KM, DeLeo FR. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol. 2012; 34:237–59.35. Ahmad-Mansour N, Loubet P, Pouget C, Dunyach-Remy C, Sotto A, Lavigne JP, et al. Staphylococcus aureus toxins: an update on their pathogenic properties and potential treatments. Toxins (Basel). 2021; 13:677.36. Spaan AN, van Strijp JA, Torres VJ. Leukocidins: Staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol. 2017; 15:435–47.37. Foster TJ, Geoghegan JA, Ganesh VK, Hook M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol. 2014; 12:49–62.38. Brandt SL, Putnam NE, Cassat JE, Serezani CH. Innate immunity to Staphylococcus aureus: evolving paradigms in soft tissue and invasive infections. J Immunol. 2018; 200:3871–80.39. Watson AR, Janik DK, Lee WT. Superantigen-induced CD4 memory T cell anergy. I. Staphylococcal enterotoxin B induces Fyn-mediated negative signaling. Cell Immunol. 2012; 276:16–25.40. Graille M, Stura EA, Corper AL, Sutton BJ, Taussig MJ, Charbonnier JB, et al. Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibody: structural basis for recognition of B-cell receptors and superantigen activity. Proc Natl Acad Sci U S A. 2000; 97:5399–404.41. Rooijakkers SH, van Wamel WJ, Ruyken M, van Kessel KP, van Strijp JA. Anti-opsonic properties of staphylokinase. Microbes Infect. 2005; 7:476–84.42. Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010; 8:623–33.43. Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012; 11:234–50.44. Urish KL, DeMuth PW, Craft DW, Haider H, Davis CM 3rd. Pulse lavage is inadequate at removal of biofilm from the surface of total knee arthroplasty materials. J Arthroplasty. 2014; 29:1128–32.45. Cheng AG, DeDent AC, Schneewind O, Missiakas D. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol. 2011; 19:225–32.46. Cheng AG, McAdow M, Kim HK, Bae T, Missiakas DM, Schneewind O. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS Pathog. 2010; 6:e1001036.47. Kavanagh N, Ryan EJ, Widaa A, Sexton G, Fennell J, O’Rourke S, et al. Staphylococcal osteomyelitis: disease progression, treatment challenges, and future directions. Clin Microbiol Rev. 2018; 31:e00084–17.48. Hofstee MI, Riool M, Terjajevs I, Thompson K, Stoddart MJ, Richards RG, et al. Three-dimensional in vitro Staphylococcus aureus abscess communities display antibiotic tolerance and protection from neutrophil clearance. Infect Immun. 2020; 88:e00293–20.49. Farnsworth CW, Schott EM, Jensen SE, Zukoski J, Benvie AM, Refaai MA, et al. Adaptive upregulation of clumping factor A (ClfA) by Staphylococcus aureus in the obese, type 2 diabetic host mediates increased virulence. Infect Immun. 2017; 85:e01005–16.50. de Mesy Bentley KL, Trombetta R, Nishitani K, Bello-Irizarry SN, Ninomiya M, Zhang L, et al. Evidence of Staphylococcus aureus deformation, proliferation, and migration in canaliculi of live cortical bone in murine models of osteomyelitis. J Bone Miner Res. 2017; 32:985–90.51. Muthukrishnan G, Beck CA, Owen JR, Xie C, Kates SL, Daiss JL. Serum antibodies against Staphylococcus aureus can prognose treatment success in patients with bone infections. J Orthop Res. 2021; 39:2169–76.52. Choi Y, Oda E, Waldman O, Sajda T, Beck C, Oh I. Next-generation sequencing for pathogen identification in infected foot ulcers. Foot Ankle Orthop. 2021; 6:24730114211026933.53. Thoendel MJ, Jeraldo PR, Greenwood-Quaintance KE, Yao JZ, Chia N, Hanssen AD, et al. Identification of prosthetic joint infection pathogens using a shotgun metagenomics approach. Clin Infect Dis. 2018; 67:1333–8.54. Namdari S, Nicholson T, Abboud J, Lazarus M, Ramsey ML, Williams G, et al. Comparative study of cultures and next-generation sequencing in the diagnosis of shoulder prosthetic joint infections. J Shoulder Elbow Surg. 2019; 28:1–8.55. Tarabichi M, Shohat N, Goswami K, Alvand A, Silibovsky R, Belden K, et al. Diagnosis of periprosthetic joint infection: the potential of next-generation sequencing. J Bone Joint Surg Am. 2018; 100:147–54.56. Tarabichi M, Shohat N, Goswami K, Parvizi J. Can next generation sequencing play a role in detecting pathogens in synovial fluid? Bone Joint J. 2018; 100-B:127–33.57. Yang Y, Lin J, Guo S, Xue X, Wang Y, Qiu S, et al. RRM2 protects against ferroptosis and is a tumor biomarker for liver cancer. Cancer Cell Int. 2020; 20:587.58. Tang B, Xu W, Wang Y, Zhu J, Wang H, Tu J, et al. Identification of critical ferroptosis regulators in lung adenocarcinoma that RRM2 facilitates tumor immune infiltration by inhibiting ferroptotic death. Clin Immunol. 2021; 232:108872.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Manifestation and Treatment of Methicillin-resistant Staphylococcus aureus Infections in Children
- Panton-Valentine Leukocidin Positive Methicillin-Susceptible Staphylococcus aureus: A Case Report of Two Pediatric Patients with Thrombotic Complications
- Clinical Observation on Staphylococcus aureus Bacteremia of Community Hospital
- Antimicrobial Resistance and Treatment Update of Skin and Soft Tissue Infections
- Emergence of Superbacteria, Vancomycin-Resistant Staphylococcus Aureus