J Korean Neurosurg Soc.
2024 Jul;67(4):418-430. 10.3340/jkns.2023.0208.
Potential Risk of Choline Alfoscerate on Isoflurane-Induced Toxicity in Primary Human Astrocytes
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, College of Medicine, Ewha Womans University, Seoul, Korea
- 2Department of Neurosurgery, Korea University Medicine, Korea University College of Medicine, Seoul, Korea
- 3Photo-Theranosis and Bioinformatics for Tumor Laboratory, Research Institute for Future Medicine, Samsung Medical Center, Seoul, Korea
- 4Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2556739
- DOI: http://doi.org/10.3340/jkns.2023.0208
Abstract
Objective
: Isoflurane, a widely used common inhalational anesthetic agent, can induce brain toxicity. The challenge lies in protecting neurologically compromised patients from neurotoxic anesthetics. Choline alfoscerate (L-α-Glycerophosphorylcholine, α-GPC) is recognized for its neuroprotective properties against oxidative stress and inflammation, but its optimal therapeutic window and indications are still under investigation. This study explores the impact of α-GPC on human astrocytes, the most abundant cells in the brain that protect against oxidative stress, under isoflurane exposure.
Methods
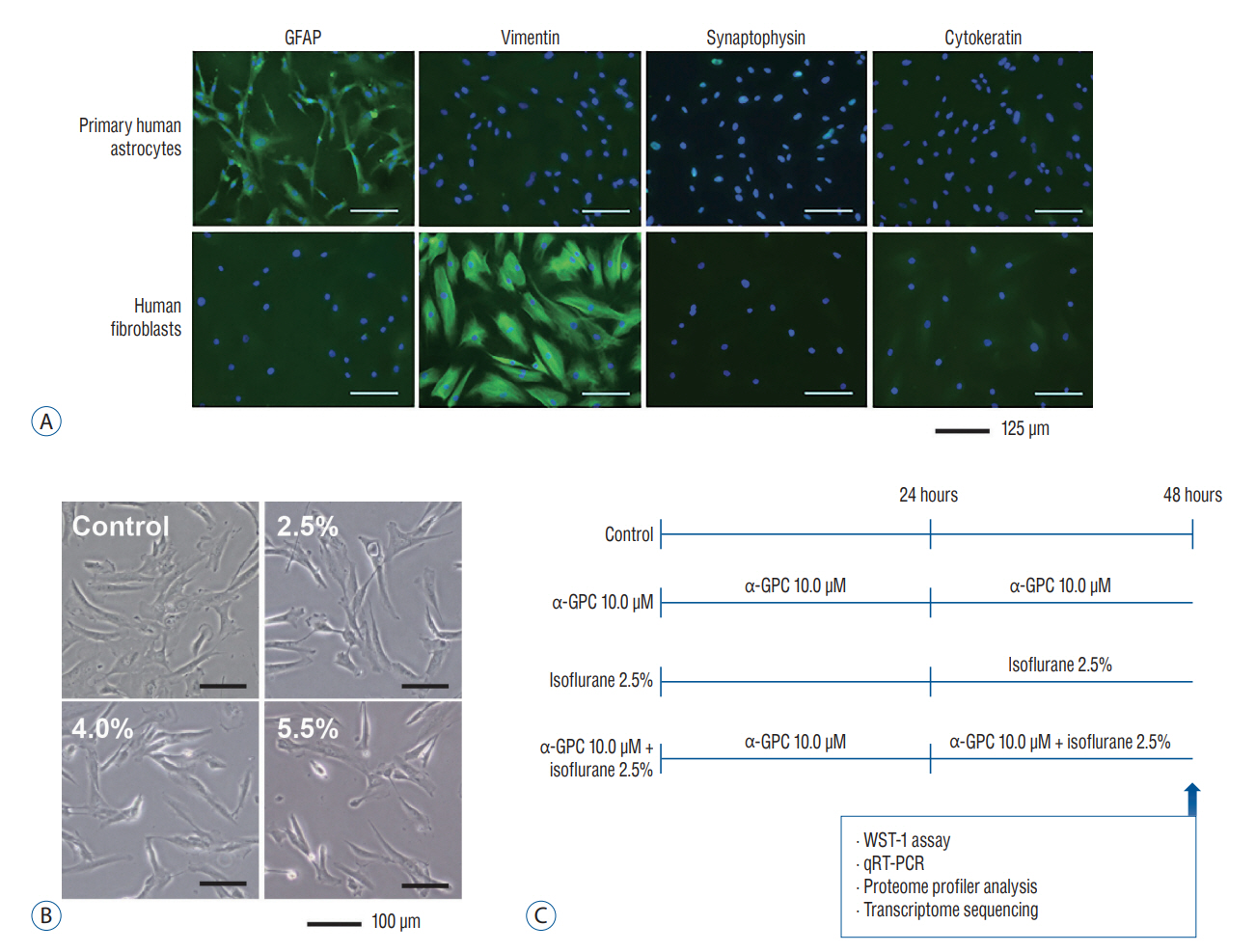
: This study was designed to examine changes in factors related to isoflurane-induced toxicity following α-GPC administration. Primary human astrocytes were pretreated with varying doses of α-GPC (ranging from 0.1 to 10.0 μM) for 24 hours prior to 2.5% isoflurane exposure. In vitro analysis of cell morphology, water-soluble tetrazolium salt-1 assay, quantitative real-time polymerase chain reaction, proteome profiler array, and transcriptome sequencing were conducted.
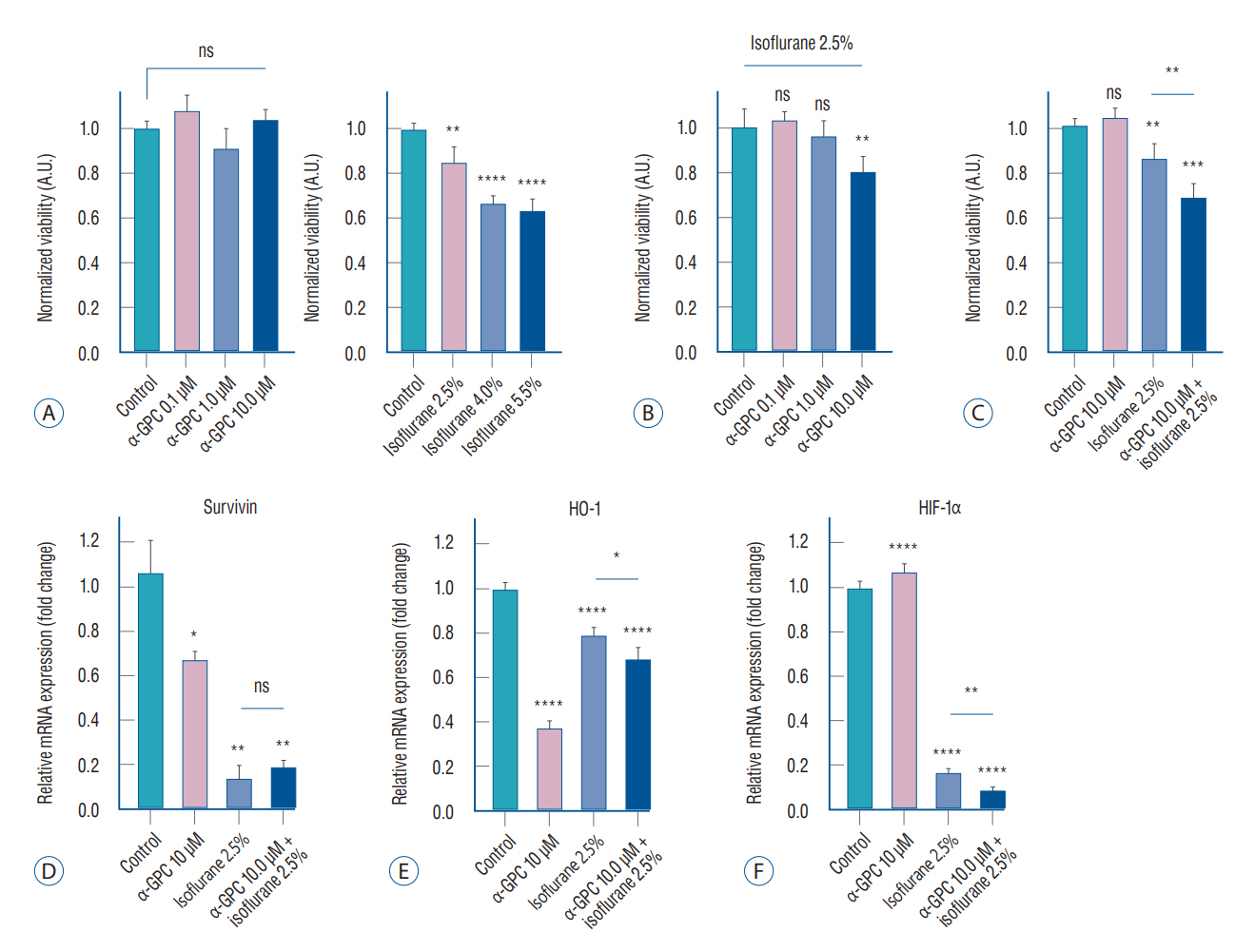
Results
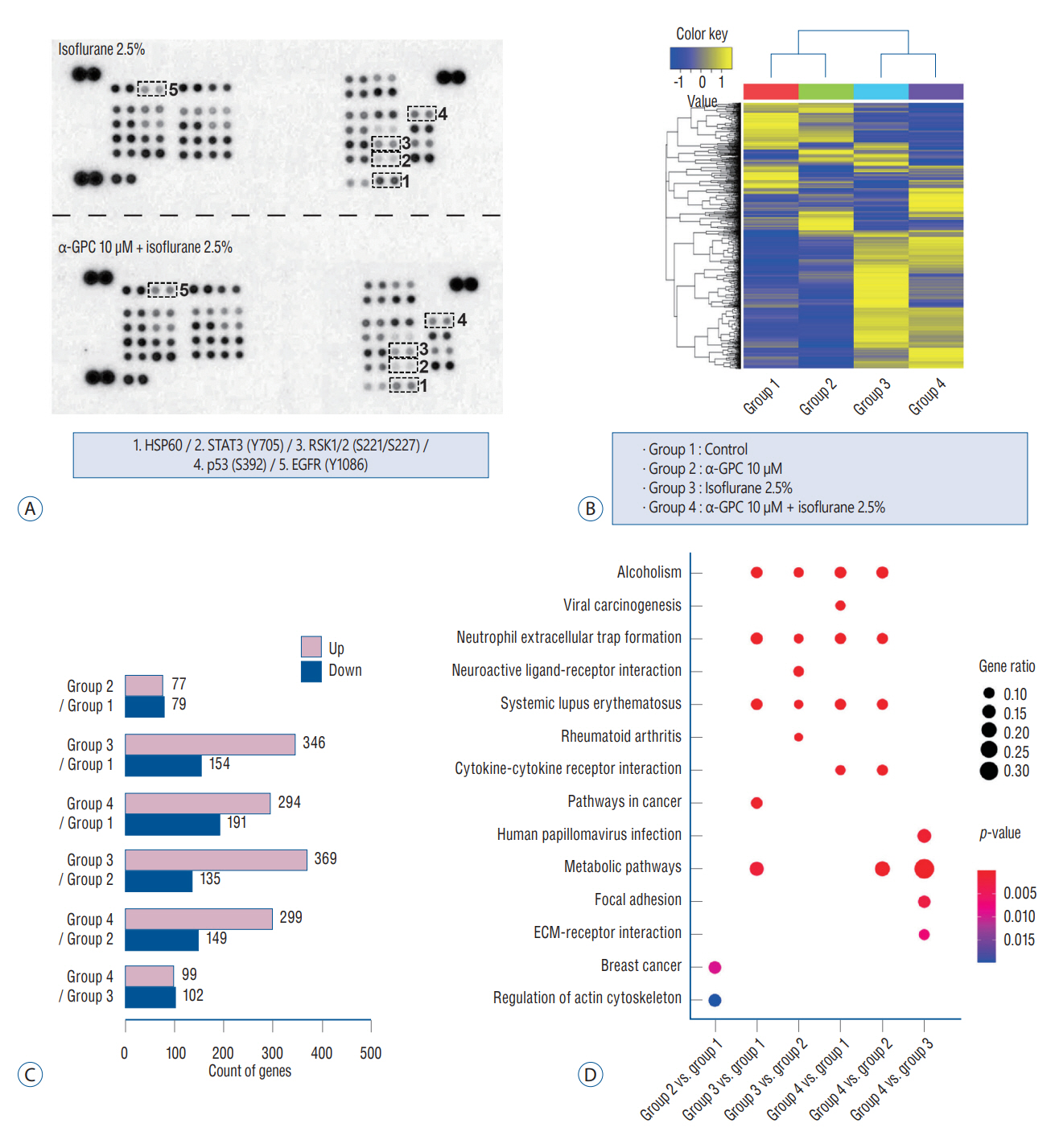
: A significant morphological damage to human astrocytes was observed in the group that had been pretreated with 10.0 mM of α-GPC and exposed to 2.5% isoflurane. A decrease in cell viability was identified in the group pretreated with 10.0 μM of α-GPC and exposed to 2.5% isoflurane compared to the group exposed only to 2.5% isoflurane. Quantitative real-time polymerase chain reaction revealed that mRNA expression of heme-oxygenase 1 and hypoxia-inducible factor-1α, which were reduced by isoflurane, was further suppressed by 10.0 μM α-GPC pretreatment. The proteome profiler array demonstrated that α-GPC pretreatment influenced a variety of factors associated with apoptosis induced by oxidative stress. Additionally, transcriptome sequencing identified pathways significantly related to changes in isoflurane-induced toxicity caused by α-GPC pretreatment.
Conclusion
: The findings suggest that α-GPC pretreatment could potentially enhance the vulnerability of primary human astrocytes to isoflurane-induced toxicity by diminishing the expression of antioxidant factors, potentially leading to amplified cell damage.
Figure
Reference
-
References
1. Bao F, Kang X, Xie Q, Wu J. HIF-α/PKM2 and PI3K-AKT pathways involved in the protection by dexmedetomidine against isoflurane or bupivacaine-induced apoptosis in hippocampal neuronal HT22 cells. Exp Ther Med. 17:63–70. 2019.
Article2. Barbagallo Sangiorgi G, Barbagallo M, Giordano M, Meli M, Panzarasa R. alpha-Glycerophosphocholine in the mental recovery of cerebral ischemic attacks. An Italian multicenter clinical trial. Ann N Y Acad Sci. 717:253–269. 1994.3. Bauer I, Raupach A. The role of heme oxygenase-1 in remote ischemic and anesthetic organ conditioning. Antioxidants (Basel). 8:403. 2019.
Article4. Burgaletto C, Di Benedetto G, Munafò A, Bernardini R, Cantarella G. Beneficial effects of choline alphoscerate on amyloid-β neurotoxicity in an in vitro model of Alzheimer's disease. Curr Alzheimer Res. 18:298–309. 2021.
Article5. Catanesi M, d'Angelo M, Antonosante A, Castelli V, Alfonsetti M, Benedetti E, et al. Neuroprotective potential of choline alfoscerate against β-amyloid injury: involvement of neurotrophic signals. Cell Biol Int. 44:1734–1744. 2020.
Article6. Chai D, Jiang H, Li Q. Isoflurane neurotoxicity involves activation of hypoxia inducible factor-1α via intracellular calcium in neonatal rodents. Brain Res. 1653:39–50. 2016.
Article7. Chen L, Chu C, Lu J, Kong X, Huang T, Cai YD. Gene ontology and KEGG pathway enrichment analysis of a drug target-based classification system. PLoS One. 10:e0126492. 2015.
Article8. Cui H, Xu Z, Qu C. Tetramethylpyrazine ameliorates isoflurane-induced cognitive dysfunction by inhibiting neuroinflammation via miR-150 in rats. Exp Ther Med. 20:3878–3887. 2020.
Article9. Culley DJ, Cotran EK, Karlsson E, Palanisamy A, Boyd JD, Xie Z, et al. Isoflurane affects the cytoskeleton but not survival, proliferation, or synaptogenic properties of rat astrocytes in vitro. Br J Anaesth 110 Suppl. 1:i19–i28. 2013.
Article10. De Jesus Moreno Moreno M. Cognitive improvement in mild to moderate Alzheimer's dementia after treatment with the acetylcholine precursor choline alfoscerate: a multicenter, double-blind, randomized, placebo-controlled trial. Clin Ther. 25:178–193. 2003.
Article11. Dringen R, Kussmaul L, Gutterer JM, Hirrlinger J, Hamprecht B. The glutathione system of peroxide detoxification is less efficient in neurons than in astroglial cells. J Neurochem. 72:2523–2530. 1999.
Article12. Evered LA, Silbert BS. Postoperative cognitive dysfunction and noncardiac surgery. Anesth Analg. 127:496–505. 2018.
Article13. Flecknell P, Lofgren JL, Dyson MC, Marini RR, Swindle MM, Wilson RP. Preanesthesia, anesthesia, analgesia, and euthanasia. In : Fox JG, Anderson LC, Otto GM, Pritchett-Corning KR, Whary MT, editors. Laboratory animal medicine. ed 3. Amsterdam: Elsevier;2015. p. 1135–1200.14. Guo H, Peng Z, Yang L, Liu X, Xie Y, Cai Y, et al. TREK-1 mediates isoflurane-induced cytotoxicity in astrocytes. BMC Anesthesiol. 17:124. 2017.
Article15. Hettiarachchi NT, Boyle JP, Dallas ML, Al-Owais MM, Scragg JL, Peers C. Heme oxygenase-1 derived carbon monoxide suppresses Aβ1-42 toxicity in astrocytes. Cell Death Dis. 8:e2884. 2017.
Article16. Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 45:D353–D361. 2017.
Article17. Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44:D457–D462. 2016.
Article18. Kobayashi K, Hayashi M, Nakano H, Fukutani Y, Sasaki K, Shimazaki M, et al. Apoptosis of astrocytes with enhanced lysosomal activity and oligodendrocytes in white matter lesions in Alzheimer's disease. Neuropathol Appl Neurobiol. 28:238–251. 2002.
Article19. Lee G, Choi S, Chang J, Choi D, Son JS, Kim K, et al. Association of L-α glycerylphosphorylcholine with subsequent stroke risk after 10 years. JAMA Netw Open. 4:e2136008. 2021.
Article20. Lee SH, Choi BY, Kim JH, Kho AR, Sohn M, Song HK, et al. Late treatment with choline alfoscerate (l-alpha glycerylphosphorylcholine, α-GPC) increases hippocampal neurogenesis and provides protection against seizure-induced neuronal death and cognitive impairment. Brain Res. 1654(Pt A):66–76. 2017.
Article21. Lee YM, Song BC, Yeum KJ. Impact of volatile anesthetics on oxidative stress and inflammation. Biomed Res Int. 2015:242709. 2015.
Article22. Li HS, Zhou YN, Li L, Li SF, Long D, Chen XL, et al. HIF-1α protects against oxidative stress by directly targeting mitochondria. Redox Biol. 25:101109. 2019.
Article23. Li QF, Zhu YS, Jiang H, Xu H, Sun Y. Heme oxygenase-1 mediates the anti-inflammatory effect of isoflurane preconditioning in LPS-stimulated macrophages. Acta Pharmacol Sin. 30:228–234. 2009.
Article24. Longnecker DE, Murphy FL. Dripps/Eckenhoff/Vandam Introduction to Anesthesia. ed 9. Philadelphia: Saunders;1997. p. 75–87.25. Lunardi N, Hucklenbruch C, Latham JR, Scarpa J, Jevtovic-Todorovic V. Isoflurane impairs immature astroglia development in vitro: the role of actin cytoskeleton. J Neuropathol Exp Neurol. 70:281–291. 2011.
Article26. Malik JA, Lone R. Heat shock proteins with an emphasis on HSP 60. Mol Biol Rep. 48:6959–6969. 2021.
Article27. Min MH, Park JH, Hur JH, Shin HC, Cho Y, Kim DD. Formulation and bioequivalence studies of choline alfoscerate tablet comparing with soft gelatin capsule in healthy male volunteers. Drug Des Devel Ther. 13:1049–1058. 2019.28. Monk TG, Price CC. Postoperative cognitive disorders. Curr Opin Crit Care. 17:376–381. 2011.
Article29. Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 108:18–30. 2008.
Article30. Needham MJ, Webb CE, Bryden DC. Postoperative cognitive dysfunction and dementia: what we need to know and do. Br J Anaesth. 119(suppl_1):i115–i125. 2017.
Article31. O'Driscoll L, Linehan R, Clynes M. Survivin: role in normal cells and in pathological conditions. Curr Cancer Drug Targets. 3:131–152. 2003.32. Parnetti L, Amenta F, Gallai V. Choline alphoscerate in cognitive decline and in acute cerebrovascular disease: an analysis of published clinical data. Mech Ageing Dev. 122:2041–2055. 2001.
Article33. Sarafian TA, Montes C, Imura T, Qi J, Coppola G, Geschwind DH, et al. Disruption of astrocyte STAT3 signaling decreases mitochondrial function and increases oxidative stress in vitro. PLoS One. 5:e9532. 2010.
Article34. Schnitzer J, Franke WW, Schachner M. Immunocytochemical demonstration of vimentin in astrocytes and ependymal cells of developing and adult mouse nervous system. J Cell Biol. 90:435–447. 1981.
Article35. Shi T, van Soest DMK, Polderman PE, Burgering BMT, Dansen TB. DNA damage and oxidant stress activate p53 through differential upstream signaling pathways. Free Radic Biol Med. 172:298–311. 2021.
Article36. Takata T, Araki S, Tsuchiya Y, Watanabe Y. Oxidative stress orchestrates MAPK and nitric-oxide synthase signal. Int J Mol Sci. 21:8750. 2020.
Article37. Tuboly E, Gáspár R, Ibor MO, Gömöri K, Kiss B, Strifler G, et al. L-alpha-glycerylphosphorylcholine can be cytoprotective or cytotoxic in neonatal rat cardiac myocytes: a double-edged sword phenomenon. Mol Cell Biochem. 460:195–203. 2019.
Article38. Vutskits L, Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci. 17:705–717. 2016.
Article39. Wei H, Liang G, Yang H, Wang Q, Hawkins B, Madesh M, et al. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. 108:251–260. 2008.
Article40. Weng MS, Chang JH, Hung WY, Yang YC, Chien MH. The interplay of reactive oxygen species and the epidermal growth factor receptor in tumor progression and drug resistance. J Exp Clin Cancer Res. 37:61. 2018.
Article41. Zhou CH, Zhang YH, Xue F, Xue SS, Chen YC, Gu T, et al. Isoflurane exposure regulates the cell viability and BDNF expression of astrocytes via upregulation of TREK-1. Mol Med Rep. 16:7305–7314. 2017.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Analysis on Prescribing Patterns of Alzheimer's Dementia Treatment and Choline Alfoscerate using HIRA Claims Data
- Effects of aluminum on choline uptake and activities of choline acetyltransferase and acetylcholinesterase in rat brain
- Human Brain Astrocytes Mediate TRAIL-mediated Apoptosis after Treatment with IFN-gamma
- Choline intake and its dietary reference values in Korea and other countries: a review
- Effect of Choline Alfoscerate on the Progression From Mild Cognitive Impairment to Dementia: Distributed Network Analysis of a Multicenter Korean Database Using a Common Data Model