Endocrinol Metab.
2024 Jun;39(3):399-406. 10.3803/EnM.2023.1895.
Osteocalcin: Beyond Bones
- Affiliations
-
- 1Department of Pediatrics, Neonatal Pathology and Metabolic Bone Diseases, Medical University of Lodz, Lodz, Poland
- KMID: 2556631
- DOI: http://doi.org/10.3803/EnM.2023.1895
Abstract
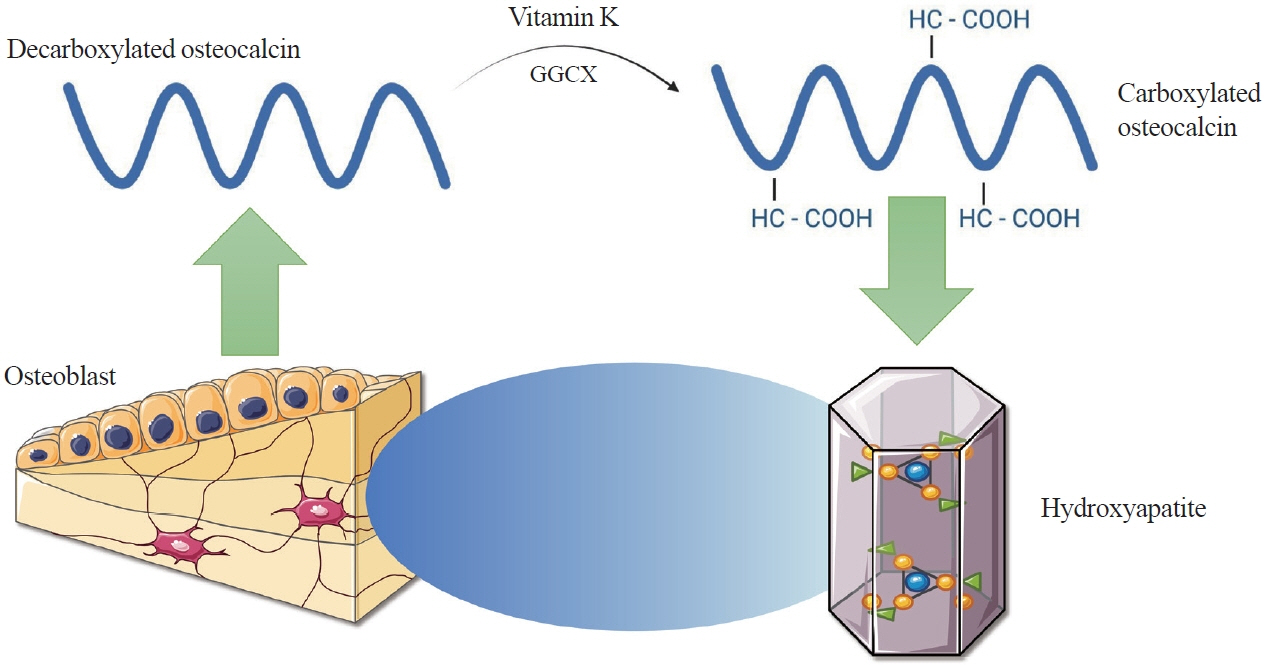
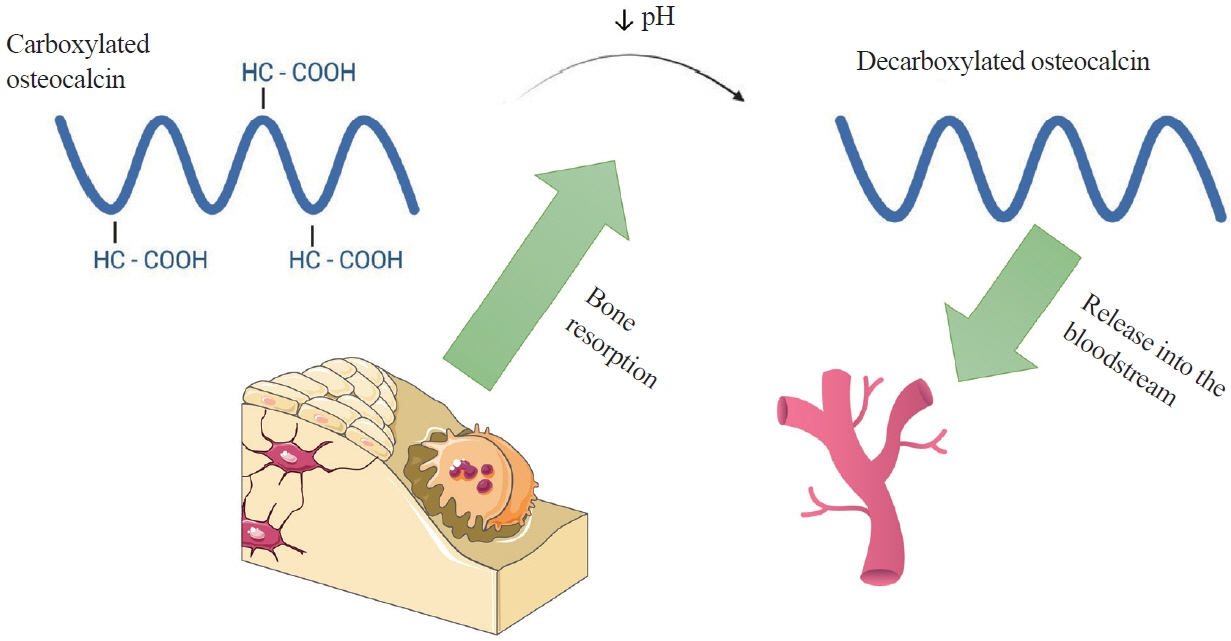
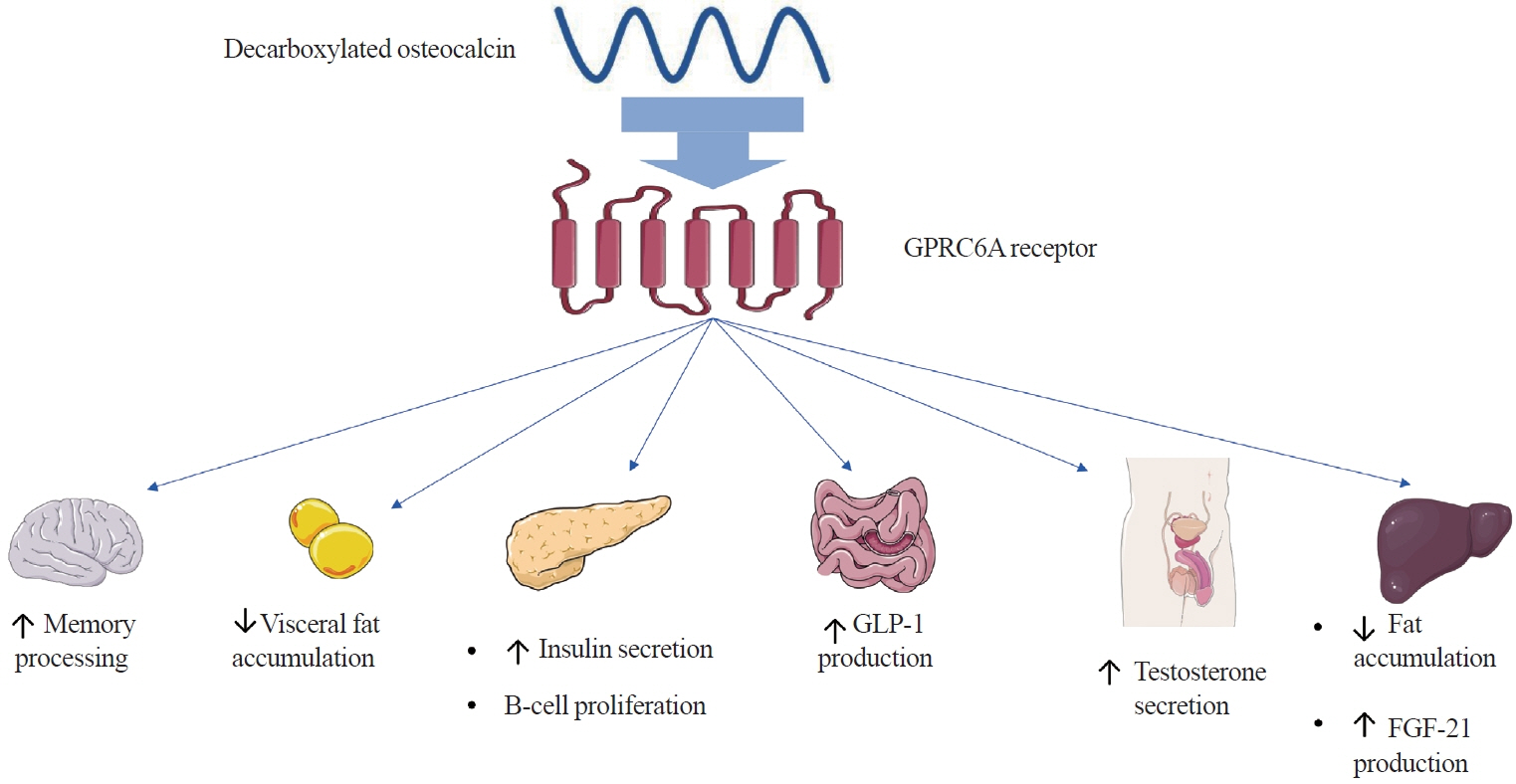
- Apart from basic roles such as supporting the body, protecting internal organs, and storing calcium, the skeletal system also performs hormonal functions. In recent years, several reports have been published on proteins secreted by bones and their impact on the homeostasis of the entire body. These proteins include fibroblast growth factor 23, sclerostin, lipocalin 2, and osteocalcin. Osteocalcin, the most abundant non-collagenous protein in bone tissue, is routinely measured as a clinical marker for diagnosing bone metabolism disorders. Its molecule undergoes numerous transformations, with decarboxylation being the critical process. Decarboxylation occurs in the acidic environment typical of bone resorption, facilitating the release of the molecule into the bloodstream and enabling its hormonal action. Decarboxylated osteocalcin promotes insulin secretion and stimulates the proliferation of pancreatic islet β-cells. It also plays a role in reducing the accumulation of visceral fat and decreasing fat storage in the liver. Furthermore, decarboxylated osteocalcin levels are inversely correlated with fasting serum glucose levels, total body fat, visceral fat area, and body mass index. Apart from its role in energy metabolism, osteocalcin affects testosterone production and the synthesis of glucagon-like peptide-1. It is also actively involved in muscle-bone crosstalk and influences cognitive function.
Keyword
Figure
Reference
-
1. Bilezikian JP, Martin TJ, Clemens TL, Rosen CJ. Principles of bone biology. 4th ed. London: Academic Press;2020. Chapter 20, Phosphorus homeostasis and related disorders. p. 469–507.2. Ho BB, Bergwitz C. FGF23 signalling and physiology. J Mol Endocrinol. 2021; 66:R23–32.
Article3. Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004; 113:561–8.
Article4. Fukumoto S. FGF23-related hypophosphatemic rickets/osteomalacia: diagnosis and new treatment. J Mol Endocrinol. 2021; 66:R57–65.
Article5. D’Onofrio L, Maddaloni E, Buzzetti R. Osteocalcin and sclerostin: background characters or main actors in cardiovascular disease? Diabetes Metab Res Rev. 2020; 36:e3217.
Article6. Wang JS, Mazur CM, Wein MN. Sclerostin and osteocalcin: candidate bone-produced hormones. Front Endocrinol (Lausanne). 2021; 12:584147.
Article7. Dirckx N, Moorer MC, Clemens TL, Riddle RC. The role of osteoblasts in energy homeostasis. Nat Rev Endocrinol. 2019; 15:651–65.
Article8. Mosialou I, Shikhel S, Liu JM, Maurizi A, Luo N, He Z, et al. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature. 2017; 543:385–90.
Article9. Manolagas SC. Osteocalcin promotes bone mineralization but is not a hormone. PLoS Genet. 2020; 16:e1008714.
Article10. Nakashima K, de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003; 19:458–66.
Article11. Atalay S, Elci A, Kayadibi H, Onder CB, Aka N. Diagnostic utility of osteocalcin, undercarboxylated osteocalcin, and alkaline phosphatase for osteoporosis in premenopausal and postmenopausal women. Ann Lab Med. 2012; 32:23–30.
Article12. Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989; 69:990–1047.
Article13. Hoang QQ, Sicheri F, Howard AJ, Yang DS. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature. 2003; 425:977–80.
Article14. Desbois C, Hogue DA, Karsenty G. The mouse osteocalcin gene cluster contains three genes with two separate spatial and temporal patterns of expression. J Biol Chem. 1994; 269:1183–90.
Article15. Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996; 382:448–52.
Article16. Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007; 130:456–69.
Article17. Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wildtype mice. Proc Natl Acad Sci U S A. 2008; 105:5266–70.18. Pi M, Chen L, Huang MZ, Zhu W, Ringhofer B, Luo J, et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS One. 2008; 3:e3858.
Article19. Pi M, Nishimoto SK, Darryl Quarles L. Explaining divergent observations regarding osteocalcin/GPRC6A endocrine signaling. Endocrinology. 2021; 162:bqab011.
Article20. Pi M, Xu F, Ye R, Nishimoto SK, Williams RW, Lu L, et al. Role of GPRC6A in regulating hepatic energy metabolism in mice. Sci Rep. 2020; 10:7216.
Article21. Mizokami A, Yasutake Y, Gao J, Matsuda M, Takahashi I, Takeuchi H, et al. Osteocalcin induces release of glucagon-like peptide-1 and thereby stimulates insulin secretion in mice. PLoS One. 2013; 8:e57375.
Article22. Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008; 60:470–512.
Article23. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006; 3:153–65.
Article24. Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011; 144:796–809.
Article25. Ferron M, Lacombe J. Regulation of energy metabolism by the skeleton: osteocalcin and beyond. Arch Biochem Biophys. 2014; 561:137–46.
Article26. Otani T, Mizokami A, Kawakubo-Yasukochi T, Takeuchi H, Inai T, Hirata M. The roles of osteocalcin in lipid metabolism in adipose tissue and liver. Adv Biol Regul. 2020; 78:100752.
Article27. Otani T, Mizokami A, Hayashi Y, Gao J, Mori Y, Nakamura S, et al. Signaling pathway for adiponectin expression in adipocytes by osteocalcin. Cell Signal. 2015; 27:532–44.
Article28. Otani T, Matsuda M, Mizokami A, Kitagawa N, Takeuchi H, Jimi E, et al. Osteocalcin triggers Fas/FasL-mediated necroptosis in adipocytes via activation of p300. Cell Death Dis. 2018; 9:1194.
Article29. Novotny SA, Warren GL, Hamrick MW. Aging and the muscle-bone relationship. Physiology (Bethesda). 2015; 30:8–16.
Article30. Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galan-Diez M, et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab. 2016; 23:1078–92.
Article31. Karsenty G, Mera P. Molecular bases of the crosstalk between bone and muscle. Bone. 2018; 115:43–9.
Article32. Mera P, Laue K, Wei J, Berger JM, Karsenty G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol Metab. 2016; 5:1042–7.
Article33. Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003; 4:638–49.
Article34. Nakamura M, Imaoka M, Takeda M. Interaction of bone and brain: osteocalcin and cognition. Int J Neurosci. 2021; 131:1115–23.
Article35. Oury F, Khrimian L, Denny CA, Gardin A, Chamouni A, Goeden N, et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013; 155:228–41.
Article36. Khrimian L, Obri A, Ramos-Brossier M, Rousseaud A, Moriceau S, Nicot AS, et al. Gpr158 mediates osteocalcin’s regulation of cognition. J Exp Med. 2017; 214:2859–73.
Article37. Moriishi T, Ozasa R, Ishimoto T, Nakano T, Hasegawa T, Miyazaki T, et al. Osteocalcin is necessary for the alignment of apatite crystallites, but not glucose metabolism, testosterone synthesis, or muscle mass. PLoS Genet. 2020; 16:e1008586.
Article38. Diegel CR, Hann S, Ayturk UM, Hu JC, Lim KE, Droscha CJ, et al. An osteocalcin-deficient mouse strain without endocrine abnormalities. PLoS Genet. 2020; 16:e1008361.
Article39. Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia. 2011; 54:1291–7.
Article40. Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010; 142:296–308.
Article41. Cousin W, Courseaux A, Ladoux A, Dani C, Peraldi P. Cloning of hOST-PTP: the only example of a protein-tyrosinephosphatase the function of which has been lost between rodent and human. Biochem Biophys Res Commun. 2004; 321:259–65.
Article42. Zhao S, Kusminski CM, Elmquist JK, Scherer PE. Leptin: less is more. Diabetes. 2020; 69:823–9.
Article43. Sharan K, Yadav VK. Hypothalamic control of bone metabolism. Best Pract Res Clin Endocrinol Metab. 2014; 28:713–23.
Article44. Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA, et al. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci U S A. 2004; 101:3258–63.
Article45. Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000; 100:197–207.
Article46. Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Myers MG Jr, et al. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008; 183:1235–42.
Article47. Iki M, Tamaki J, Fujita Y, Kouda K, Yura A, Kadowaki E, et al. Serum undercarboxylated osteocalcin levels are inversely associated with glycemic status and insulin resistance in an elderly Japanese male population: Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Study. Osteoporos Int. 2012; 23:761–70.
Article48. Ma XY, Chen FQ, Hong H, Lv XJ, Dong M, Wang QY. The relationship between serum osteocalcin concentration and glucose and lipid metabolism in patients with type 2 diabetes mellitus: the role of osteocalcin in energy metabolism. Ann Nutr Metab. 2015; 66:110–6.
Article49. Kanazawa I, Yamaguchi T, Tada Y, Yamauchi M, Yano S, Sugimoto T. Serum osteocalcin level is positively associated with insulin sensitivity and secretion in patients with type 2 diabetes. Bone. 2011; 48:720–5.
Article50. Zheng WB, Hu J, Zhao DC, Zhou BN, Wang O, Jiang Y, et al. The role of osteocalcin in regulation of glycolipid metabolism and muscle function in children with osteogenesis imperfecta. Front Endocrinol (Lausanne). 2022; 13:898645.
Article51. Wang JW, Tang QY, Ruan HJ, Cai W. Relation between serum osteocalcin levels and body composition in obese children. J Pediatr Gastroenterol Nutr. 2014; 58:729–32.
Article52. Takashi Y, Ishizu M, Mori H, Miyashita K, Sakamoto F, Katakami N, et al. Circulating osteocalcin as a bone-derived hormone is inversely correlated with body fat in patients with type 1 diabetes. PLoS One. 2019; 14:e0216416.
Article53. Alfadda AA, Masood A, Shaik SA, Dekhil H, Goran M. Association between osteocalcin, metabolic syndrome, and cardiovascular risk factors: role of total and undercarboxylated osteocalcin in patients with type 2 diabetes. Int J Endocrinol. 2013; 2013:197519.
Article54. Liu X, Yeap BB, Brock KE, Levinger I, Golledge J, Flicker L, et al. Associations of osteocalcin forms with metabolic syndrome and its individual components in older men: the health in men study. J Clin Endocrinol Metab. 2021; 106:e3506–18.
Article55. Tan A, Gao Y, Yang X, Zhang H, Qin X, Mo L, et al. Low serum osteocalcin level is a potential marker for metabolic syndrome: results from a Chinese male population survey. Metabolism. 2011; 60:1186–92.
Article56. Levinger I, Scott D, Nicholson GC, Stuart AL, Duque G, McCorquodale T, et al. Undercarboxylated osteocalcin, muscle strength and indices of bone health in older women. Bone. 2014; 64:8–12.
Article57. Bradburn S, McPhee JS, Bagley L, Sipila S, Stenroth L, Narici MV, et al. Association between osteocalcin and cognitive performance in healthy older adults. Age Ageing. 2016; 45:844–9.
Article58. Kord-Varkaneh H, Djafarian K, Khorshidi M, Shab-Bidar S. Association between serum osteocalcin and body mass index: a systematic review and meta-analysis. Endocrine. 2017; 58:24–32.
Article59. Liu C, Wo J, Zhao Q, Wang Y, Wang B, Zhao W. Association between serum total osteocalcin level and type 2 diabetes mellitus: a systematic review and meta-analysis. Horm Metab Res. 2015; 47:813–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Integrative Physiology: Defined Novel Metabolic Roles of Osteocalcin
- An overview of the endocrine functions of osteocalcin
- Osteocalcin Response to Calcium Restricted Diet for the Selective Therapy of Hypercalciuria
- A Comparative Study of Osteocalcin Measured by Radioimmunoassay in Normal
- Osteocalcin expression in primary bone tumors: in situ hybridization and immunohistochemical study