Anat Cell Biol.
2024 Jun;57(2):155-162. 10.5115/acb.24.003.
The underlying mechanism of calcium toxicityinduced autophagic cell death and lysosomal degradation in early stage of cerebral ischemia
- Affiliations
-
- 1Department of Anatomy, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
- 2Excellence in Osteology Research and Training Center (ORTC), Chaing Mai University, Chiang Mai, Thailand
- KMID: 2556559
- DOI: http://doi.org/10.5115/acb.24.003
Abstract
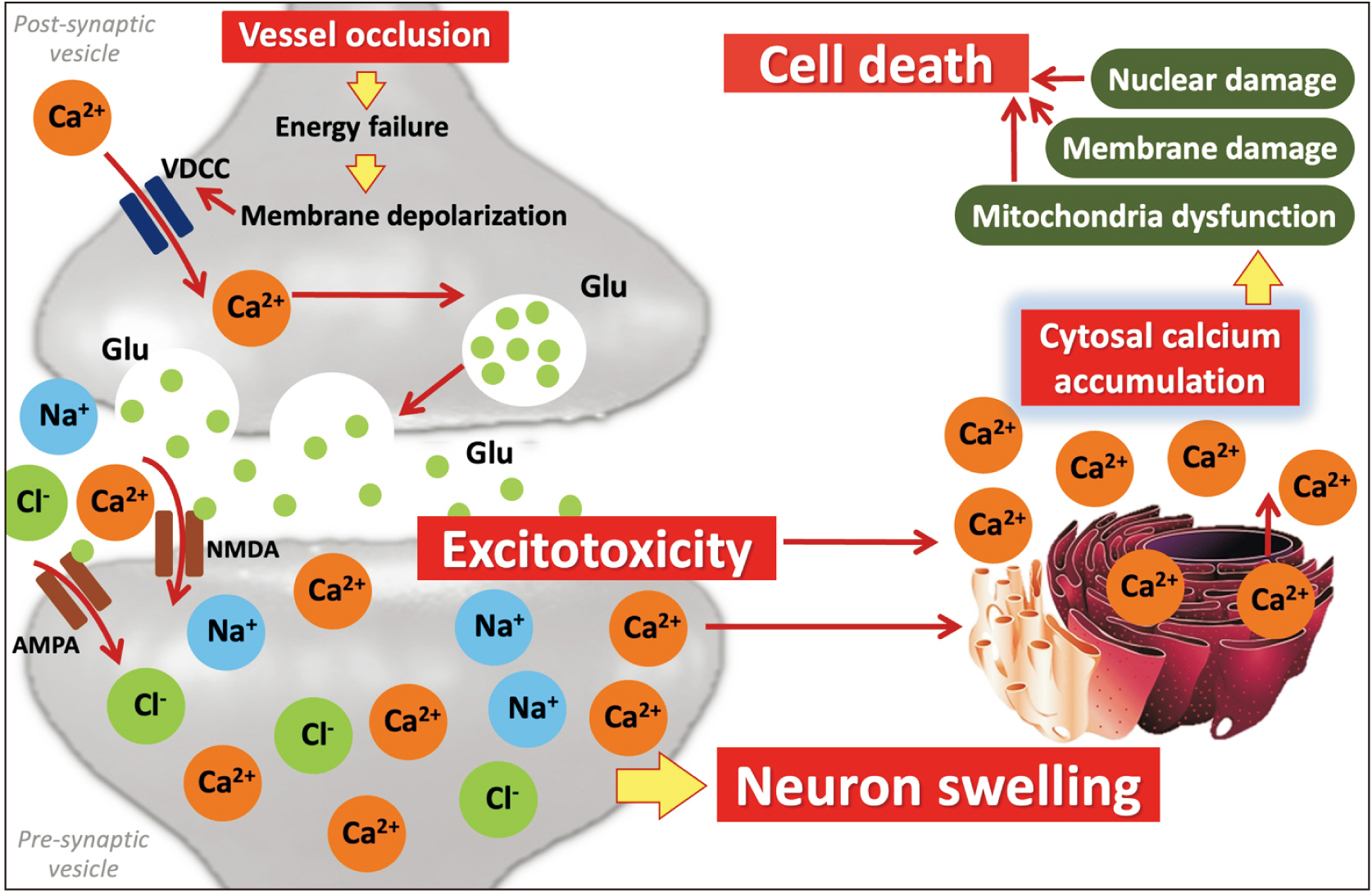
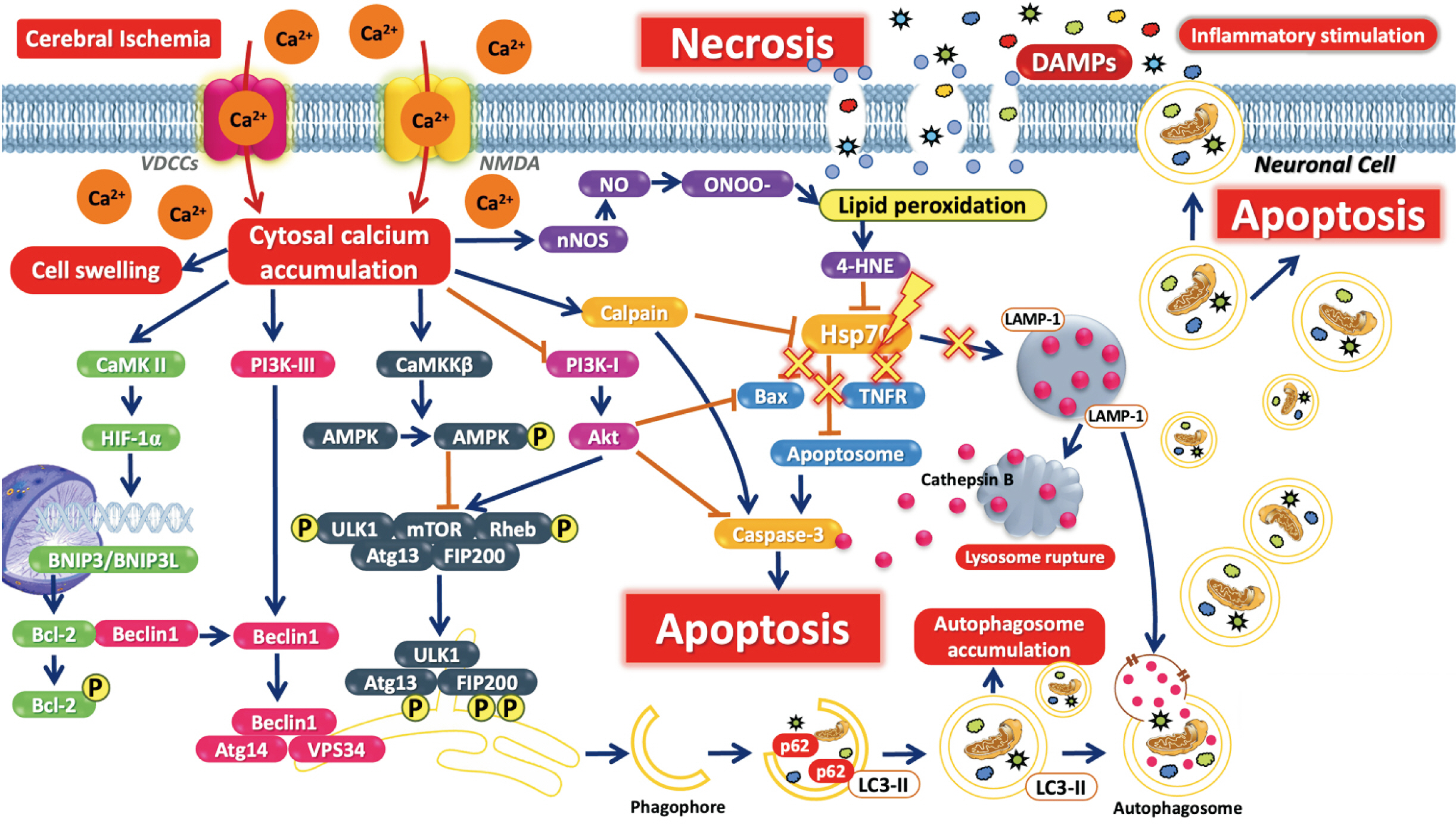
- Cerebral ischemia is the important cause of worldwide disability and mortality, that is one of the obstruction of blood vessels supplying to the brain. In early stage, glutamate excitotoxicity and high level of intracellular calcium (Ca2+ ) are the major processes which can promote many downstream signaling involving in neuronal death and brain tissue damaging. Moreover, autophagy, the reusing of damaged cell organelles, is affected in early ischemia. Under ischemic conditions, autophagy plays an important role to maintain energy of the brain and its function. In the other hand, over intracellular Ca2+ accumulation triggers excessive autophagic process and lysosomal degradation leading to autophagic process impairment which finally induce neuronal death. This article reviews the association between intracellular Ca2+ and autophagic process in acute stage of ischemic stroke.
Figure
Reference
-
References
1. Saini V, Guada L, Yavagal DR. 2021; Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. 97(20 Suppl 2):S6–16. DOI: 10.1212/WNL.0000000000012781. PMID: 34785599.
Article2. Feigin VL, Brainin M, Norrving B, Martins S, Sacco RL, Hacke W, Fisher M, Pandian J, Lindsay P. 2022; World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke. 17:18–29. Erratum in: Int J Stroke 2022;17:478. DOI: 10.1177/17474930211065917. PMID: 34986727.
Article3. Donnan GA, Fisher M, Macleod M, Davis SM. 2008; Stroke. Lancet. 371:1612–23. DOI: 10.1016/S0140-6736(08)60694-7. PMID: 18468545.
Article4. Johnstone VP, Shultz SR, Yan EB, O'Brien TJ, Rajan R. 2014; The acute phase of mild traumatic brain injury is characterized by a distance-dependent neuronal hypoactivity. J Neurotrauma. 31:1881–95. DOI: 10.1089/neu.2014.3343. PMID: 24927383. PMCID: PMC4224042.
Article5. DeSai C, Hays Shapshak A. 2023. Apr. 3. Cerebral ischemia [Internet]. StatPearls;Available from: https://www.ncbi.nlm.nih.gov/books/NBK560510/.6. Anderson JA. 2014; The golden hour Performing an acute ischemic stroke workup. Nurse Pract. 39:22–9. quiz 29–30. DOI: 10.1097/01.NPR.0000452974.46311.0f. PMID: 25083767.
Article7. Advani R, Naess H, Kurz MW. 2017; The golden hour of acute ischemic stroke. Scand J Trauma Resusc Emerg Med. 25:54. DOI: 10.1186/s13049-017-0398-5. PMID: 28532498. PMCID: PMC5440901.
Article8. Singh V, Mishra VN, Chaurasia RN, Joshi D, Pandey V. 2019; Modes of calcium regulation in ischemic neuron. Indian J Clin Biochem. 34:246–53. DOI: 10.1007/s12291-019-00838-9. PMID: 31391713. PMCID: PMC6660593.
Article9. Chen W, Sun Y, Liu K, Sun X. 2014; Autophagy: a double-edged sword for neuronal survival after cerebral ischemia. Neural Regen Res. 9:1210–6. DOI: 10.4103/1673-5374.135329. PMID: 25206784. PMCID: PMC4146291.
Article10. Davis GW. 2020; Not fade away: mechanisms of neuronal ATP homeostasis. Neuron. 105:591–3. DOI: 10.1016/j.neuron.2020.01.024. PMID: 32078791.
Article11. Agnati LF, Guidolin D, Cervetto C, Maura G, Marcoli M. 2023; Brain structure and function: insights from chemical neuroanatomy. Life (Basel). 13:940. DOI: 10.3390/life13040940. PMID: 37109469. PMCID: PMC10142941.
Article12. Bertrand PP. 2003; ATP and sensory transduction in the enteric nervous system. Neuroscientist. 9:243–60. DOI: 10.1177/1073858403253768. PMID: 12934708.
Article13. Clarke SG, Scarnati MS, Paradiso KG. 2016; Neurotransmitter release can be stabilized by a mechanism that prevents voltage changes near the end of action potentials from affecting calcium currents. J Neurosci. 36:11559–72. DOI: 10.1523/JNEUROSCI.0066-16.2016. PMID: 27911759. PMCID: PMC5125219.
Article14. Zbili M, Rama S, Debanne D. 2016; Dynamic control of neurotransmitter release by presynaptic potential. Front Cell Neurosci. 10:278. DOI: 10.3389/fncel.2016.00278. PMID: 27994539. PMCID: PMC5136543.
Article15. Sifat AE, Nozohouri S, Archie SR, Chowdhury EA, Abbruscato TJ. 2022; Brain energy metabolism in ischemic stroke: effects of smoking and diabetes. Int J Mol Sci. 23:8512. DOI: 10.3390/ijms23158512. PMID: 35955647. PMCID: PMC9369264.
Article16. Liu F, Lu J, Manaenko A, Tang J, Hu Q. 2018; Mitochondria in ischemic stroke: new insight and implications. Aging Dis. 9:924–37. DOI: 10.14336/AD.2017.1126. PMID: 30271667. PMCID: PMC6147588.
Article17. Suhail M. 2010; Na, K-ATPase: ubiquitous multifunctional transmembrane protein and its relevance to various pathophysiological conditions. J Clin Med Res. 2:1–17. DOI: 10.4021/jocmr2010.02.263w. PMID: 22457695. PMCID: PMC3299169.
Article18. Shen Z, Xiang M, Chen C, Ding F, Wang Y, Shang C, Xin L, Zhang Y, Cui X. 2022; Glutamate excitotoxicity: potential therapeutic target for ischemic stroke. Biomed Pharmacother. 151:113125. DOI: 10.1016/j.biopha.2022.113125. PMID: 35609367.
Article19. Belov Kirdajova D, Kriska J, Tureckova J, Anderova M. 2020; Ischemia-triggered glutamate excitotoxicity from the perspective of glial cells. Front Cell Neurosci. 14:51. DOI: 10.3389/fncel.2020.00051. PMID: 32265656. PMCID: PMC7098326.
Article20. de Lores Arnaiz GR, Ordieres MG. 2014; Brain Na(+), K(+)-ATPase activity in aging and disease. Int J Biomed Sci. 10:85–102. DOI: 10.59566/IJBS.2014.10085. PMID: 25018677. PMCID: PMC4092085.
Article21. Wang F, Xie X, Xing X, Sun X. 2022; Excitatory synaptic transmission in ischemic stroke: a new outlet for classical neuroprotective strategies. Int J Mol Sci. 23:9381. DOI: 10.3390/ijms23169381. PMID: 36012647. PMCID: PMC9409263.
Article22. Nishizawa Y. 2001; Glutamate release and neuronal damage in ischemia. Life Sci. 69:369–81. DOI: 10.1016/S0024-3205(01)01142-0. PMID: 11459428.
Article23. Franco R, Rivas-Santisteban R, Lillo J, Camps J, Navarro G, Reyes-Resina I. 2021; 5-hydroxytryptamine, glutamate, and ATP: much more than neurotransmitters. Front Cell Dev Biol. 9:667815. DOI: 10.3389/fcell.2021.667815. PMID: 33937270. PMCID: PMC8083958.
Article24. Mahmoud S, Gharagozloo M, Simard C, Gris D. 2019; Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells. 8:184. DOI: 10.3390/cells8020184. PMID: 30791579. PMCID: PMC6406900.
Article25. Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. 2010; Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 62:405–96. Erratum in: Pharmacol Rev 2014;66:1141. DOI: 10.1124/pr.109.002451. PMID: 20716669. PMCID: PMC2964903.
Article26. Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. 1995; Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 15:961–73. DOI: 10.1016/0896-6273(95)90186-8. PMID: 7576644.
Article27. Mattson MP. 2003; Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 3:65–94. DOI: 10.1385/NMM:3:2:65. PMID: 12728191.
Article28. Hellas JA, Andrew RD. 2021; Neuronal swelling: a non-osmotic consequence of spreading depolarization. Neurocrit Care. 35(Suppl 2):112–34. DOI: 10.1007/s12028-021-01326-w. PMID: 34498208. PMCID: PMC8536653.
Article29. Kahle KT, Simard JM, Staley KJ, Nahed BV, Jones PS, Sun D. 2009; Molecular mechanisms of ischemic cerebral edema: role of electroneutral ion transport. Physiology (Bethesda). 24:257–65. DOI: 10.1152/physiol.00015.2009. PMID: 19675357.
Article30. Akins PT, Atkinson RP. Glutamate AMPA receptor antagonist treatment for ischaemic stroke. Curr Med Res Opin. 2002; 18(Suppl 2):s9–13. DOI: 10.1185/030079902125000660. PMID: 12365832.
Article31. Besancon E, Guo S, Lok J, Tymianski M, Lo EH. 2008; Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci. 29:268–75. DOI: 10.1016/j.tips.2008.02.003. PMID: 18384889.
Article32. von Engelhardt J, Coserea I, Pawlak V, Fuchs EC, Köhr G, Seeburg PH, Monyer H. 2007; Excitotoxicity in vitro by NR2A- and NR2B-containing NMDA receptors. Neuropharmacology. 53:10–7. DOI: 10.1016/j.neuropharm.2007.04.015. PMID: 17570444.
Article33. Zhou X, Ding Q, Chen Z, Yun H, Wang H. 2013; Involvement of the GluN2A and GluN2B subunits in synaptic and extrasynaptic N-methyl-D-aspartate receptor function and neuronal excitotoxicity. J Biol Chem. 288:24151–9. DOI: 10.1074/jbc.M113.482000. PMID: 23839940. PMCID: PMC3745357.
Article34. Lai TW, Zhang S, Wang YT. 2014; Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol. 115:157–88. DOI: 10.1016/j.pneurobio.2013.11.006. PMID: 24361499.
Article35. Wu QJ, Tymianski M. 2018; Targeting NMDA receptors in stroke: new hope in neuroprotection. Mol Brain. 11:15. DOI: 10.1186/s13041-018-0357-8. PMID: 29534733. PMCID: PMC5851248.
Article36. Lujan B, Liu X, Wan Q. 2012; Differential roles of GluN2A- and GluN2B-containing NMDA receptors in neuronal survival and death. Int J Physiol Pathophysiol Pharmacol. 4:211–8.37. Franchini L, Carrano N, Di Luca M, Gardoni F. 2020; Synaptic GluN2A-containing NMDA receptors: from physiology to pathological synaptic plasticity. Int J Mol Sci. 21:1538. DOI: 10.3390/ijms21041538. PMID: 32102377. PMCID: PMC7073220.
Article38. Li Y, Cheng X, Liu X, Wang L, Ha J, Gao Z, He X, Wu Z, Chen A, Jewell LL, Sun Y. 2022; Treatment of cerebral ischemia through NMDA receptors: metabotropic signaling and future directions. Front Pharmacol. 13:831181. DOI: 10.3389/fphar.2022.831181. PMID: 35264964. PMCID: PMC8900870.
Article39. Sun Y, Zhang L, Chen Y, Zhan L, Gao Z. 2015; Therapeutic targets for cerebral ischemia based on the signaling pathways of the GluN2B C terminus. Stroke. 46:2347–53. DOI: 10.1161/STROKEAHA.115.009314. PMID: 26173725.
Article40. Picón-Pagès P, Garcia-Buendia J, Muñoz FJ. 2019; Functions and dysfunctions of nitric oxide in brain. Biochim Biophys Acta Mol Basis Dis. 1865:1949–67. DOI: 10.1016/j.bbadis.2018.11.007. PMID: 30500433.
Article41. Sulaiman Alsaadi M. 2019; Role of DAPK1 in neuronal cell death, survival and diseases in the nervous system. Int J Dev Neurosci. 74:11–7. DOI: 10.1016/j.ijdevneu.2019.02.003. PMID: 30763607.
Article42. Kim N, Chen D, Zhou XZ, Lee TH. 2019; Death-associated protein kinase 1 phosphorylation in neuronal cell death and neurodegenerative disease. Int J Mol Sci. 20:3131. DOI: 10.3390/ijms20133131. PMID: 31248062. PMCID: PMC6651373.
Article43. Lee JH, Rho SB, Chun T. 2005; Programmed cell death 6 (PDCD6) protein interacts with death-associated protein kinase 1 (DAPk1): additive effect on apoptosis via caspase-3 dependent pathway. Biotechnol Lett. 27:1011–5. DOI: 10.1007/s10529-005-7869-x. PMID: 16132846.
Article44. Nair S, Hagberg H, Krishnamurthy R, Thornton C, Mallard C. 2013; Death associated protein kinases: molecular structure and brain injury. Int J Mol Sci. 14:13858–72. DOI: 10.3390/ijms140713858. PMID: 23880846. PMCID: PMC3742222.
Article45. Ludhiadch A, Sharma R, Muriki A, Munshi A. 2022; Role of calcium homeostasis in ischemic stroke: a review. CNS Neurol Disord Drug Targets. 21:52–61. DOI: 10.2174/1871527320666210212141232. PMID: 33583386.
Article46. Cross JL, Meloni BP, Bakker AJ, Lee S, Knuckey NW. 2010; Modes of neuronal calcium entry and homeostasis following cerebral ischemia. Stroke Res Treat. 2010:316862. DOI: 10.4061/2010/316862. PMID: 21052549. PMCID: PMC2968719.
Article47. Liu J, Liu MC, Wang KK. 2008; Calpain in the CNS: from synaptic function to neurotoxicity. Sci Signal. 1:re1. DOI: 10.1126/stke.114re1.
Article48. Bevers MB, Neumar RW. 2008; Mechanistic role of calpains in postischemic neurodegeneration. J Cereb Blood Flow Metab. 28:655–73. DOI: 10.1038/sj.jcbfm.9600595. PMID: 18073773.
Article49. Cheng SY, Wang SC, Lei M, Wang Z, Xiong K. 2018; Regulatory role of calpain in neuronal death. Neural Regen Res. 13:556–62. DOI: 10.4103/1673-5374.228762. PMID: 29623944. PMCID: PMC5900522.
Article50. Yamakawa H, Banno Y, Nakashima S, Yoshimura S, Sawada M, Nishimura Y, Nozawa Y, Sakai N. 2001; Crucial role of calpain in hypoxic PC12 cell death: calpain, but not caspases, mediates degradation of cytoskeletal proteins and protein kinase C-alpha and -delta. Neurol Res. 23:522–30. DOI: 10.1179/016164101101198776. PMID: 11474809.51. Bano D, Nicotera P. 2007; Ca2+ signals and neuronal death in brain ischemia. Stroke. 38(2 Suppl):674–6. DOI: 10.1161/01.STR.0000256294.46009.29. PMID: 17261713.52. Xu W, Wong TP, Chery N, Gaertner T, Wang YT, Baudry M. 2007; Calpain-mediated mGluR1alpha truncation: a key step in excitotoxicity. Neuron. 53:399–412. DOI: 10.1016/j.neuron.2006.12.020. PMID: 17270736.
Article53. Reggiori F, Klionsky DJ. 2002; Autophagy in the eukaryotic cell. Eukaryot Cell. 1:11–21. DOI: 10.1128/EC.01.1.11-21.2002. PMID: 12455967. PMCID: PMC118053.
Article54. Mehrpour M, Esclatine A, Beau I, Codogno P. 2010; Overview of macroautophagy regulation in mammalian cells. Cell Res. 20:748–62. DOI: 10.1038/cr.2010.82. PMID: 20548331.
Article55. Yim WW, Mizushima N. 2020; Lysosome biology in autophagy. Cell Discov. 6:6. DOI: 10.1038/s41421-020-0141-7. PMID: 32047650. PMCID: PMC7010707.
Article56. Dunlop EA, Tee AR. 2014; mTOR and autophagy: a dynamic relationship governed by nutrients and energy. Semin Cell Dev Biol. 36:121–9. DOI: 10.1016/j.semcdb.2014.08.006. PMID: 25158238.
Article57. Wong PM, Puente C, Ganley IG, Jiang X. 2013; The ULK1 complex: sensing nutrient signals for autophagy activation. Autophagy. 9:124–37. DOI: 10.4161/auto.23323. PMID: 23295650. PMCID: PMC3552878.58. McKnight NC, Zhenyu Y. 2013; Beclin 1, an essential component and master regulator of PI3K-III in health and disease. Curr Pathobiol Rep. 1:231–8. DOI: 10.1007/s40139-013-0028-5. PMID: 24729948. PMCID: PMC3979578.
Article59. Tanida I, Ueno T, Kominami E. 2004; LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 36:2503–18. DOI: 10.1016/j.biocel.2004.05.009. PMID: 15325588. PMCID: PMC7129593.
Article60. Shibutani ST, Yoshimori T. 2014; A current perspective of autophagosome biogenesis. Cell Res. 24:58–68. DOI: 10.1038/cr.2013.159. PMID: 24296784. PMCID: PMC3879706.
Article61. Settembre C, Fraldi A, Medina DL, Ballabio A. 2013; Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 14:283–96. DOI: 10.1038/nrm3565. PMID: 23609508. PMCID: PMC4387238.
Article62. Peng L, Hu G, Yao Q, Wu J, He Z, Law BY, Hu G, Zhou X, Du J, Wu A, Yu L. 2022; Microglia autophagy in ischemic stroke: a double-edged sword. Front Immunol. 13:1013311. DOI: 10.3389/fimmu.2022.1013311. PMID: 36466850. PMCID: PMC9708732.
Article63. Rami A, Langhagen A, Steiger S. 2008; Focal cerebral ischemia induces upregulation of Beclin 1 and autophagy-like cell death. Neurobiol Dis. 29:132–41. DOI: 10.1016/j.nbd.2007.08.005. PMID: 17936001.
Article64. Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, Han F, Fukunaga K, Qin ZH. 2008; Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy. 4:762–9. DOI: 10.4161/auto.6412. PMID: 18567942.
Article65. Russo R, Berliocchi L, Adornetto A, Varano GP, Cavaliere F, Nucci C, Rotiroti D, Morrone LA, Bagetta G, Corasaniti MT. 2011; Calpain-mediated cleavage of Beclin-1 and autophagy deregulation following retinal ischemic injury in vivo. Cell Death Dis. 2:e144. DOI: 10.1038/cddis.2011.29. PMID: 21490676. PMCID: PMC3122060.66. Liu Y, Che X, Zhang H, Fu X, Yao Y, Luo J, Yang Y, Cai R, Yu X, Yang J, Zhou MS. 2021; CAPN1 (calpain1)-mediated impairment of autophagic flux contributes to cerebral ischemia-induced neuronal damage. Stroke. 52:1809–21. DOI: 10.1161/STROKEAHA.120.032749. PMID: 33874744.
Article67. Kim J, Yang G, Kim Y, Kim J, Ha J. 2016; AMPK activators: mechanisms of action and physiological activities. Exp Mol Med. 48:e224. DOI: 10.1038/emm.2016.16. PMID: 27034026. PMCID: PMC4855276.
Article68. Mayer MP, Bukau B. 2005; Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 62:670–84. DOI: 10.1007/s00018-004-4464-6. PMID: 15770419. PMCID: PMC2773841.
Article69. Sharma D, Masison DC. 2009; Hsp70 structure, function, regulation and influence on yeast prions. Protein Pept Lett. 16:571–81. DOI: 10.2174/092986609788490230. PMID: 19519514. PMCID: PMC2746719.
Article70. Rosenzweig R, Nillegoda NB, Mayer MP, Bukau B. 2019; The Hsp70 chaperone network. Nat Rev Mol Cell Biol. 20:665–80. DOI: 10.1038/s41580-019-0133-3. PMID: 31253954.
Article71. Balogi Z, Multhoff G, Jensen TK, Lloyd-Evans E, Yamashima T, Jäättelä M, Harwood JL, Vígh L. 2019; Hsp70 interactions with membrane lipids regulate cellular functions in health and disease. Prog Lipid Res. 74:18–30. DOI: 10.1016/j.plipres.2019.01.004. PMID: 30710597.
Article72. Lee SH, Kim M, Yoon BW, Kim YJ, Ma SJ, Roh JK, Lee JS, Seo JS. 2001; Targeted hsp70.1 disruption increases infarction volume after focal cerebral ischemia in mice. Stroke. 32:2905–12. DOI: 10.1161/hs1201.099604. PMID: 11739994.
Article73. Kim JY, Kim N, Zheng Z, Lee JE, Yenari MA. 2016; 70-kDa heat shock protein downregulates dynamin in experimental stroke: a new therapeutic target? Stroke. 47:2103–11. DOI: 10.1161/STROKEAHA.116.012763. PMID: 27387989. PMCID: PMC4961549.
Article74. Zhou XY, Luo Y, Zhu YM, Liu ZH, Kent TA, Rong JG, Li W, Qiao SG, Li M, Ni Y, Ishidoh K, Zhang HL. 2017; Inhibition of autophagy blocks cathepsins-tBid-mitochondrial apoptotic signaling pathway via stabilization of lysosomal membrane in ischemic astrocytes. Cell Death Dis. 8:e2618. DOI: 10.1038/cddis.2017.34. PMID: 28206988. PMCID: PMC5386481.
Article75. Villalpando Rodriguez GE, Torriglia A. 2013; Calpain 1 induce lysosomal permeabilization by cleavage of lysosomal associated membrane protein 2. Biochim Biophys Acta. 1833:2244–53. DOI: 10.1016/j.bbamcr.2013.05.019. PMID: 23747342.
Article76. Qin AP, Zhang HL, Qin ZH. 2008; Mechanisms of lysosomal proteases participating in cerebral ischemia-induced neuronal death. Neurosci Bull. 24:117–23. DOI: 10.1007/s12264-008-0117-3. PMID: 18369392. PMCID: PMC5552511.
Article77. Terasaki Y, Liu Y, Hayakawa K, Pham LD, Lo EH, Ji X, Arai K. 2014; Mechanisms of neurovascular dysfunction in acute ischemic brain. Curr Med Chem. 21:2035–42. DOI: 10.2174/0929867321666131228223400. PMID: 24372202. PMCID: PMC4066327.
Article78. Lipton P. 2013; Lysosomal membrane permeabilization as a key player in brain ischemic cell death: a "lysosomocentric" hypothesis for ischemic brain damage. Transl Stroke Res. 4:672–84. DOI: 10.1007/s12975-013-0301-2. PMID: 24323421.
Article79. Li J, McCullough LD. 2010; Effects of AMP-activated protein kinase in cerebral ischemia. J Cereb Blood Flow Metab. 30:480–92. DOI: 10.1038/jcbfm.2009.255. PMID: 20010958. PMCID: PMC2852687.
Article80. Chen H, Kim GS, Okami N, Narasimhan P, Chan PH. 2011; NADPH oxidase is involved in post-ischemic brain inflammation. Neurobiol Dis. 42:341–8. DOI: 10.1016/j.nbd.2011.01.027. PMID: 21303700. PMCID: PMC3079796.
Article81. Yamashima T, Mathivanan A, Dazortsava MY, Sakai S, Kurimoto S, Zhu H, Funaki N, Liang H, Hullin-Matsuda F, Kobayashi T, Akatsu H, Takahashi H, Minabe Y. 2014; Calpain-mediated Hsp70.1 cleavage in monkey CA1 after ischemia induces similar - lysosomal vesiculosis' to Alzheimer neurons. J Alzheimers Dis Parkinsonism. 4:139. DOI: 10.4172/2161-0460.1000139.82. Yamashima T. 2012; Hsp70.1 and related lysosomal factors for necrotic neuronal death. J Neurochem. 120:477–94. DOI: 10.1111/j.1471-4159.2011.07596.x. PMID: 22118687.
Article83. Koriyama Y, Furukawa A. 2016; HSP70 cleavage-induced photoreceptor cell death caused by N-methyl-N-nitrosourea. Neural Regen Res. 11:1758–9. DOI: 10.4103/1673-5374.194721. PMID: 28123413. PMCID: PMC5204225.
Article84. Wei R, Wang J, Xu Y, Yin B, He F, Du Y, Peng G, Luo B. 2015; Probenecid protects against cerebral ischemia/reperfusion injury by inhibiting lysosomal and inflammatory damage in rats. Neuroscience. 301:168–77. DOI: 10.1016/j.neuroscience.2015.05.070. PMID: 26047730.
Article85. Tontchev AB, Yamashima T. 1999; Ischemic delayed neuronal death: role of the cysteine proteases calpain and cathepsins. Neuropathology. 19:356–65. DOI: 10.1046/j.1440-1789.1999.00259.x.
Article86. Chaitanya GV, Babu PP. 2008; Activation of calpain, cathepsin-b and caspase-3 during transient focal cerebral ischemia in rat model. Neurochem Res. 33:2178–86. DOI: 10.1007/s11064-007-9567-7. PMID: 18338260.
Article87. Lang-Rollin IC, Rideout HJ, Noticewala M, Stefanis L. 2003; Mechanisms of caspase-independent neuronal death: energy depletion and free radical generation. J Neurosci. 23:11015–25. DOI: 10.1523/JNEUROSCI.23-35-11015.2003. PMID: 14657158. PMCID: PMC6741034.
Article88. Chen J, Hu R, Liao H, Zhang Y, Lei R, Zhang Z, Zhuang Y, Wan Y, Jin P, Feng H, Wan Q. 2017; A non-ionotropic activity of NMDA receptors contributes to glycine-induced neuroprotection in cerebral ischemia-reperfusion injury. Sci Rep. 7:3575. DOI: 10.1038/s41598-017-03909-0. PMID: 28620235. PMCID: PMC5472592.
Article89. Chen M, Lu TJ, Chen XJ, Zhou Y, Chen Q, Feng XY, Xu L, Duan WH, Xiong ZQ. 2008; Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke. 39:3042–8. DOI: 10.1161/STROKEAHA.108.521898. PMID: 18688011.
Article90. Kotwal A, Ramalingaiah AH, Shukla D, Radhakrishnan M, Konar SK, inivasaiah B Sr, Chakrabarti D, Sundaram M. 2022; Role of nimodipine and milrinone in delayed cerebral ischemia. World Neurosurg. 166:e285–93. DOI: 10.1016/j.wneu.2022.06.150. PMID: 35843579.
Article91. Liu S, Liu C, Xiong L, Xie J, Huang C, Pi R, Huang Z, Li L. 2021; Icaritin alleviates glutamate-induced neuronal damage by inactivating GluN2B-containing NMDARs through the ERK/DAPK1 pathway. Front Neurosci. 15:525615. DOI: 10.3389/fnins.2021.525615. PMID: 33692666. PMCID: PMC7937872.
Article92. Wang X, Fang Y, Huang Q, Xu P, Lenahan C, Lu J, Zheng J, Dong X, Shao A, Zhang J. 2021; An updated review of autophagy in ischemic stroke: from mechanisms to therapies. Exp Neurol. 340:113684. DOI: 10.1016/j.expneurol.2021.113684. PMID: 33676918.
Article93. Yuan J, Zhang Z, Ni J, Wu X, Yan H, Xu J, Zhao Q, Yuan H, Yang L. 2023; Acupuncture for autophagy in animal models of middle cerebral artery occlusion: a systematic review and meta-analysis protocol. PLoS One. 18:e0281956. DOI: 10.1371/journal.pone.0281956. PMID: 36812222. PMCID: PMC9946199.
Article94. Lu X, Zhang J, Ding Y, Wu J, Chen G. 2022; Novel therapeutic strategies for ischemic stroke: recent insights into autophagy. Oxid Med Cell Longev. 2022:3450207. DOI: 10.1155/2022/3450207. PMID: 35720192. PMCID: PMC9200548.
Article95. Ahsan A, Liu M, Zheng Y, Yan W, Pan L, Li Y, Ma S, Zhang X, Cao M, Wu Z, Hu W, Chen Z, Zhang X. 2021; Natural compounds modulate the autophagy with potential implication of stroke. Acta Pharm Sin B. 11:1708–20. DOI: 10.1016/j.apsb.2020.10.018. PMID: 34386317. PMCID: PMC8343111.
Article96. Yao Y, Ji Y, Ren J, Liu H, Khanna R, Sun L. 2021; Inhibition of autophagy by CRMP2-derived peptide ST2-104 (R9-CBD3) via a CaMKKβ/AMPK/mTOR pathway contributes to ischemic postconditioning-induced neuroprotection against cerebral ischemia-reperfusion injury. Mol Brain. 14:123. DOI: 10.1186/s13041-021-00836-0. PMID: 34362425. PMCID: PMC8344221.
Article97. Wicha P, Onsa-Ard A, Chaichompoo W, Suksamrarn A, Tocharus C. 2020; Vasorelaxant and antihypertensive effects of neferine in rats: an in vitro and in vivo study. Planta Med. 86:496–504. DOI: 10.1055/a-1123-7852. PMID: 32219782.
Article98. Sengking J, Oka C, Wicha P, Yawoot N, Tocharus J, Chaichompoo W, Suksamrarn A, Tocharus C. 2021; Neferine protects against brain damage in permanent cerebral ischemic rat associated with autophagy suppression and AMPK/mTOR regulation. Mol Neurobiol. 58:6304–15. DOI: 10.1007/s12035-021-02554-z. PMID: 34498225.
Article99. Liu CW, Liao KH, Tseng H, Wu CM, Chen HY, Lai TW. 2020; Hypothermia but not NMDA receptor antagonism protects against stroke induced by distal middle cerebral arterial occlusion in mice. PLoS One. 15:e0229499. DOI: 10.1371/journal.pone.0229499. PMID: 32126102. PMCID: PMC7053748.
Article100. Hu WW, Du Y, Li C, Song YJ, Zhang GY. 2008; Neuroprotection of hypothermia against neuronal death in rat hippocampus through inhibiting the increased assembly of GluR6-PSD95-MLK3 signaling module induced by cerebral ischemia/reperfusion. Hippocampus. 18:386–97. DOI: 10.1002/hipo.20402. PMID: 18172894.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Laser Capture Microdissection Reveals Specific Genes Related to Purkinje Cell Death in the Leaner Mice
- Autophagy: a lysosomal degradation process for cellular homeostasis and its relationship with oral squamous cell carcinoma
- Pathogenic Role of Autophagy in Rheumatic Diseases
- Neuronal Autophagy: A Housekeeper or a Fighter in Neuronal Cell Survival?
- Focal cerebral ischemic injury decreases calbindin expression in brain tissue and HT22 cells