Restor Dent Endod.
2024 May;49(2):e22. 10.5395/rde.2024.49.e22.
The prevalence of apical periodontitis in patients prior to hematopoietic cell transplantation: a systematic review
- Affiliations
-
- 1Department of Endodontics, School of Dentistry, Federal University of Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil
- KMID: 2556551
- DOI: http://doi.org/10.5395/rde.2024.49.e22
Abstract
Objectives
This systematic review addressed the question: “What is the prevalence of apical periodontitis in patients prior to hematopoietic cell transplantation?”
Materials and Methods
A systematic search was conducted in MEDLINE/PubMed, Cochrane Library, Scopus, Web of Science, Embase, and Grey Literature Report. Eligibility criteria were based on the condition, content, and population strategy: the condition was the radiographic prevalence of apical periodontitis, the content comprised patients scheduled for hematopoietic stem cell transplantation, and the population consisted of adult and pediatric patients. The revised Risk of Bias in Nonrandomized Studies of Exposure tool was used to assess the quality of studies. The Grading Recommendations Assessments, Development, and Evaluation (GRADE) tool was used to assess the quality of evidence.
Results
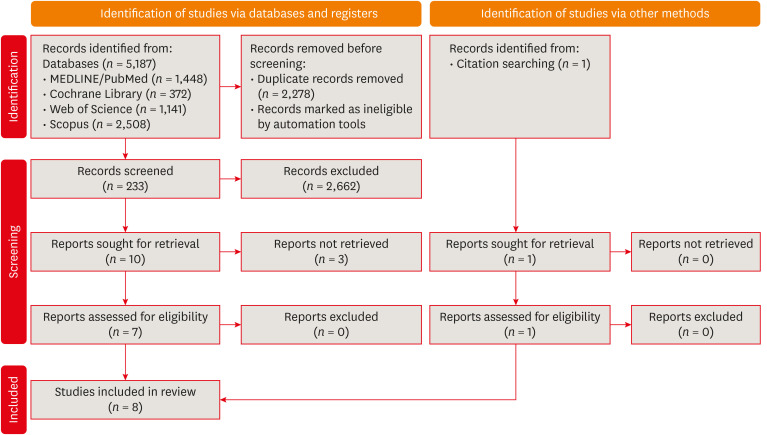
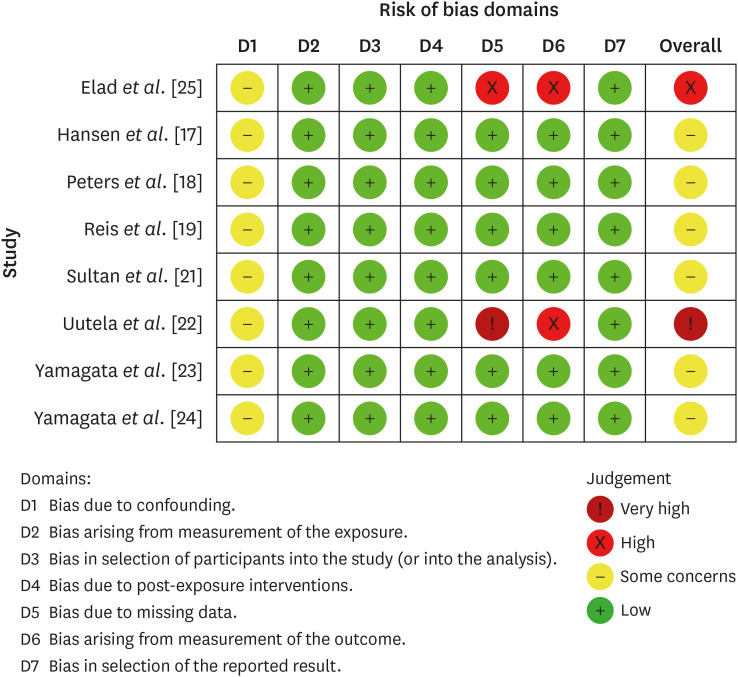
Eight studies were included in this review. The average number of patients with apical periodontitis was 15.65% (range, 2.1%–43.34%). One study was classified as having a very high risk of bias, 1 with a high risk of bias, and 6 with some concern for bias. GRADE analysis showed a very low certainty of evidence. Significant limitations concerning the absence of control over confounding variables were identified.
Conclusions
With the caveat of the very low quality of evidence in the studies reviewed, there was a low to moderate prevalence of apical periodontitis in patients prior to undergoing hematopoietic cell transplantation.
Keyword
Figure
Reference
-
1. Sureda A, Bader P, Cesaro S, Dreger P, Duarte RF, Dufour C, et al. Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplant. 2015; 50:1037–1056. PMID: 25798672.2. Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006; 354:1813–1826. PMID: 16641398.3. Elad S, Raber-Durlacher JE, Brennan MT, Saunders DP, Mank AP, Zadik Y, et al. Basic oral care for hematology-oncology patients and hematopoietic stem cell transplantation recipients: a position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT). Support Care Cancer. 2015; 23:223–236. PMID: 25189149.4. Lucas VS, Roberts GJ, Beighton D. Oral health of children undergoing allogeneic bone marrow transplantation. Bone Marrow Transplant. 1998; 22:801–808. PMID: 9827979.5. Tibúrcio-Machado CS, Michelon C, Zanatta FB, Gomes MS, Marin JA, Bier CA. The global prevalence of apical periodontitis: a systematic review and meta-analysis. Int Endod J. 2021; 54:712–735. PMID: 33378579.6. Haapasalo M, Udnæs T, Endal U. Persistent, recurrent, and acquired infection of the root canal system post‐treatment. Endod Topics. 2003; 6:29–56.7. Nair PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004; 15:348–381. PMID: 15574679.8. Gomes BP, Herrera DR. Etiologic role of root canal infection in apical periodontitis and its relationship with clinical symptomatology. Braz Oral Res. 2018; 32(Supplement 1):e69. PMID: 30365610.9. Graber CJ, de Almeida KN, Atkinson JC, Javaheri D, Fukuda CD, Gill VJ, et al. Dental health and viridans streptococcal bacteremia in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2001; 27:537–542. PMID: 11313689.10. Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.2. updated February 2021. cited June 7, 2023. Available from: https://training.cochrane.org/handbook.11. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid-Based Healthc. 2015; 13:147–153. PMID: 26317388.12. Munn Z, Stern C, Aromataris E, Lockwood C, Jordan Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol. 2018; 18:5. PMID: 29316881.13. ROBINS-E Development Group. Risk of Bias in Non-randomized Studies - of Exposure (ROBINS-E). Launch version, 1 June 2022. updated June 1, 2022. cited June 7, 2023. Available from: https://www.riskofbias.info/welcome/robins-e-tool.14. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011; 64:383–394. PMID: 21195583.15. Bogusławska-Kapała A, Struzycka I, Hałaburda K. Current attitudes to the elimination of infection foci from the oral cavity of adult patients qualified for allogeneic hematopoietic stem cell transplantation. Pol Merkuriusz Lek. 2013; 35:305–308.16. Donker AE, van Merkesteyn JP, Bredius RG, van Weel-Sipman MH. Value of panoramic radiographs in paediatric pre-bone marrow transplantation oral evaluation. Int J Oral Maxillofac Surg. 2002; 31:170–172. PMID: 12102415.17. Hansen HJ, Estilo C, Owosho A, Solano AK, Randazzo J, Huryn J, et al. Dental status and risk of odontogenic complication in patients undergoing hematopoietic stem cell transplant. Support Care Cancer. 2021; 29:2231–2238. PMID: 32901321.18. Peters E, Monopoli M, Woo SB, Sonis S. Assessment of the need for treatment of postendodontic asymptomatic periapical radiolucencies in bone marrow transplant recipients. Oral Surg Oral Med Oral Pathol. 1993; 76:45–48. PMID: 8351120.19. Reis TC, Bortolotti F, Innocentini LMAR, Ferrari TC, Ricz HMA, Cunha RLG, et al. Assessment of oral health condition in recipients of allogeneic hematopoietic cell transplantation. Hematol Transfus Cell Ther. 2022; 44:549–554. PMID: 34090846.20. Skallsjö K, von Bültzingslöwen I, Hasséus B, Johansson JE, Öhman J, Raber-Durlacher JE, et al. Oral health in patients scheduled for hematopoietic stem cell transplantation in the Orastem study. PLoS One. 2023; 18:e0285615. PMID: 37200298.21. Sultan AS, Zimering Y, Petruzziello G, Alyea EP 3rd, Antin JH, Soiffer RJ, et al. Oral health status and risk of bacteremia following allogeneic hematopoietic cell transplantation. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017; 124:253–260. PMID: 28823316.22. Uutela P, Passweg J, Halter J, Weiger R, Waltimo T, Mauramo M. Common oral diseases in allogeneic haematopoietic stem cell transplantation (HSCT) recipients pre-HSCT. Eur J Haematol. 2019; 102:351–356. PMID: 30632215.23. Yamagata K, Onizawa K, Yanagawa T, Hasegawa Y, Kojima H, Nagasawa T, et al. A prospective study to evaluate a new dental management protocol before hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006; 38:237–242. PMID: 16850033.24. Yamagata K, Onizawa K, Yoshida H, Yamagata K, Kojima Y, Koike K, et al. Dental management of pediatric patients undergoing hematopoietic stem cell transplant. Pediatr Hematol Oncol. 2006; 23:541–548. PMID: 16928649.25. Elad S, Garfunkel AA, Or R, Michaeli E, Shapira MY, Galili D. Time limitations and the challenge of providing infection-preventing dental care to hematopoietic stem-cell transplantation patients. Support Care Cancer. 2003; 11:674–677. PMID: 12883964.26. Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). J Clin Epidemiol. 2011; 64:407–415. PMID: 21247734.27. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. J Clin Epidemiol. 2011; 64:1294–1302. PMID: 21803546.28. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence--indirectness. J Clin Epidemiol. 2011; 64:1303–1310. PMID: 21802903.29. Murad MH, Mustafa RA, Schünemann HJ, Sultan S, Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. Evid Based Med. 2017; 22:85–87. PMID: 28320705.30. Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011; 64:1311–1316. PMID: 21802902.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association between cigarette smoking and the prevalence of postendodontic periapical pathology: a systematic review and meta-analysis
- Opening the era of in vivo xenotransplantation model for hematopoietic stem cell transplantation
- Hematopoietic Stem Cell Transplantation
- Hematopoietic Stem Cell Transplantation in Inborn Error of Metabolism
- Effects of adjacent periodontitis on osseointegrated dental implants