Restor Dent Endod.
2024 May;49(2):e12. 10.5395/rde.2024.49.e12.
Impact of different agitation methods on smear layer cleaning of mesial canals with accentuated curvature
- Affiliations
-
- 1Department of Operative Dentistry, Endodontics and Dental Materials, Bauru School of Dentistry, University of São Paulo – USP, Bauru, SP, Brazil
- KMID: 2556541
- DOI: http://doi.org/10.5395/rde.2024.49.e12
Abstract
Objectives
This study evaluated the impact of different methods of irrigant agitation on smear layer removal in the apical third of curved mesial canals of 3 dimensionally (D) printed mandibular molars.
Materials and Methods
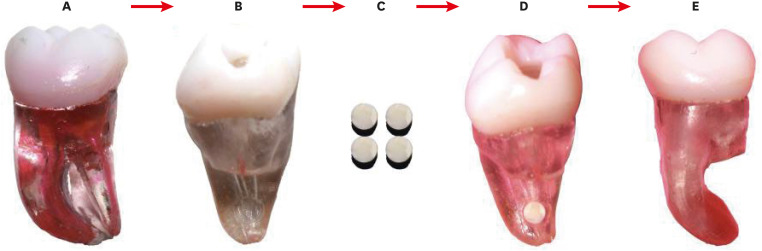
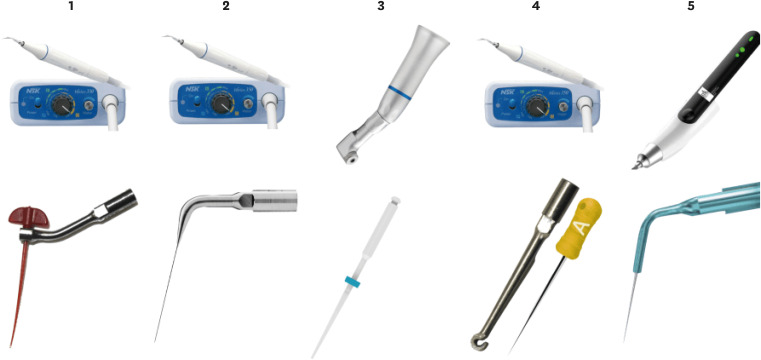
Sixty 3D-printed mandibular second molars were used, presenting a 70° curvature and a Vertucci type II configuration in the mesial root. A round cavity was cut 2 mm from the apex using a trephine of 2 mm in diameter, 60 bovine dentin disks were made, and a smear layer was formed. The dentin disks had the adaptation checked in the apical third of the teeth with wax. The dentin disks were evaluated in environmental scanning electron microscope before and after the following irrigant agitation methods: G1(PIK Ultrasonic Tip), G2 (Passive Ultrasonic Irrigation with Irrisonic– PUI), G3 (Easy Clean), G4 (HBW Ultrasonic Tip), G5 (Ultramint X Ultrasonic tip), and G6 (conventional irrigation-CI) (n = 10). All groups were irrigated with 2.5% sodium hypochlorite and 17% ethylenediaminetetraacetic acid.
Results
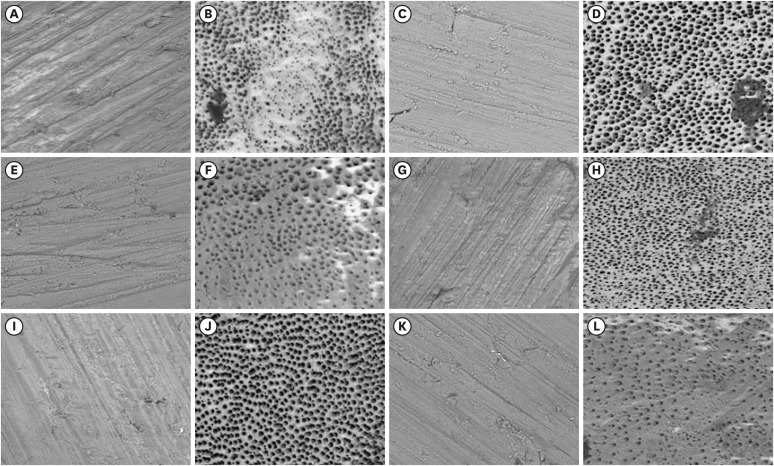
All dentin disks were 100% covered by the smear layer before treatment, and all groups significantly reduced the percentage of the smear layer after treatment. After the irrigation protocols, the Ultra-X group showed the lowest coverage percentage, statistically differing from the conventional, PIK, and HBW groups (p < 0.05). There was no significant difference among Ultramint X, PUI-Irrisonic, and Easy Clean (p > 0.05). None of the agitation methods could remove the smear layer altogether.
Conclusions
Ultramint X resulted in the most significant number of completely clean specimens.
Figure
Reference
-
1. Zehnder M. Root canal irrigants. J Endod. 2006; 32:389–398. PMID: 16631834.2. Virdee SS, Seymour DW, Farnell D, Bhamra G, Bhakta S. Efficacy of irrigant activation techniques in removing intracanal smear layer and debris from mature permanent teeth: a systematic review and meta-analysis. Int Endod J. 2018; 51:605–621. PMID: 29178166.3. Torabinejad M, Handysides R, Khademi AA, Bakland LK. Clinical implications of the smear layer in endodontics: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 94:658–666. PMID: 12464887.4. Violich DR, Chandler NP. The smear layer in endodontics - a review. Int Endod J. 2010; 43:2–15. PMID: 20002799.5. Shahravan A, Haghdoost AA, Adl A, Rahimi H, Shadifar F. Effect of smear layer on sealing ability of canal obturation: a systematic review and meta-analysis. J Endod. 2007; 33:96–105. PMID: 17258623.6. Zancan RF, Di Maio A, Tomson PL, Duarte MA, Camilleri J. The presence of smear layer affects the antimicrobial action of root canal sealers. Int Endod J. 2021; 54:1369–1382. PMID: 33763882.7. Portenier I, Haapasalo H, Rye A, Waltimo T, Ørstavik D, Haapasalo M. Inactivation of root canal medicaments by dentine, hydroxylapatite and bovine serum albumin. Int Endod J. 2001; 34:184–188. PMID: 12193263.8. Urban K, Donnermeyer D, Schäfer E, Bürklein S. Canal cleanliness using different irrigation activation systems: a SEM evaluation. Clin Oral Investig. 2017; 21:2681–2687.9. Orlowski NB, Schimdt TF, Teixeira CD, Garcia LD, Savaris JM, Tay FR, et al. Smear layer removal using passive ultrasonic irrigation and different concentrations of sodium hypochlorite. J Endod. 2020; 46:1738–1744. PMID: 32721483.10. van der Sluis LW, Versluis M, Wu MK, Wesselink PR. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J. 2007; 40:415–426. PMID: 17442017.11. Duque JA, Duarte MA, Canali LC, Zancan RF, Vivan RR, Bernardes RA, et al. Comparative effectiveness of new mechanical irrigant agitating devices for debris removal from the canal and isthmus of mesial roots of mandibular molars. J Endod. 2017; 43:326–331. PMID: 27989584.12. Machado R, da Silva I, Comparin D, de Mattos BA, Alberton LR, da Silva Neto UX. Smear layer removal by passive ultrasonic irrigation and 2 new mechanical methods for activation of the chelating solution. Restor Dent Endod. 2021; 46:e11. PMID: 33680900.13. Güven Y, Ali A, Arslan H. Efficiency of Endosonic Blue, EDDY, Ultra X and EndoActivator in the removal of calcium hydroxide paste from root canals. Aust Endod J. 2022; 48:32–36. PMID: 34939722.14. Coaguila-Llerena H, Lazo-Quezada G, Teves A, Zevallos-Chávez M, Faria G. Removal of separated instruments from unfavourable locations: case reports using the HBW ultrasonic ring or a surgical approach. Aust Endod J. 2023; 49:358–364. PMID: 35932460.15. Teves A, Blanco D, Casaretto M, Torres J, Alvarado DE, Coaguila-Llerena H, et al. Multispecies biofilm removal by XP-endo Finisher and passive ultrasonic irrigation: a scanning electron microscopy study. Aust Endod J. 2022; 48:91–97. PMID: 34310795.16. Parente JM, Loushine RJ, Susin L, Gu L, Looney SW, Weller RN, et al. Root canal debridement using manual dynamic agitation or the EndoVac for final irrigation in a closed system and an open system. Int Endod J. 2010; 43:1001–1012. PMID: 20722753.17. Yassen GH, Platt JA, Hara AT. Bovine teeth as substitute for human teeth in dental research: a review of literature. J Oral Sci. 2011; 53:273–282. PMID: 21959653.18. Tanaka JL, Medici Filho E, Salgado JA, Salgado MA, Moraes LC, Moraes ME, et al. Comparative analysis of human and bovine teeth: radiographic density. Braz Oral Res. 2008; 22:346–351. PMID: 19148391.19. Falla-Sotelo FO, Rizzutto MA, Tabacniks MH, Added IN, Barbosa I, Markarian RA, et al. Analysis and discussion of trace elements in teeth of different animal species. Braz J Phys. 2005; 35:761–762.20. Teruel JD, Alcolea A, Hernández A, Ruiz AJ. Comparison of chemical composition of enamel and dentine in human, bovine, porcine and ovine teeth. Arch Oral Biol. 2015; 60:768–775. PMID: 25766469.21. Martins Justo A, Abreu da Rosa R, Santini MF, Cardoso Ferreira MB, Pereira JR, Húngaro Duarte MA, et al. Effectiveness of final irrigant protocols for debris removal from simulated canal irregularities. J Endod. 2014; 40:2009–2014. PMID: 25266470.22. Ordinola-Zapata R, Bramante CM, Duarte MA, Cavenago BC, Jaramillo D, Versiani MA. Shaping ability of Reciproc and TF adaptive systems in severely curved canals of rapid microCT-based prototyping molar replicas. J Appl Oral Sci. 2014; 22:509–515. PMID: 24918662.23. Anderson J, Wealleans J, Ray J. Endodontic applications of 3D printing. Int Endod J. 2018; 51:1005–1018. PMID: 29486052.24. Reymus M, Fotiadou C, Kessler A, Heck K, Hickel R, Diegritz C. 3D printed replicas for endodontic education. Int Endod J. 2019; 52:123–130. PMID: 29900562.25. Boutsioukis C, Arias-Moliz MT, Chávez de Paz LE. A critical analysis of research methods and experimental models to study irrigants and irrigation systems. Int Endod J. 2022; 55(Supplement 2):295–329. PMID: 35171506.26. Gambarini G, Laszkiewicz J. A scanning electron microscopic study of debris and smear layer remaining following use of GT rotary instruments. Int Endod J. 2002; 35:422–427. PMID: 12059912.27. Rossi-Fedele G, Doğramaci EJ, Guastalli AR, Steier L, de Figueiredo JA. Antagonistic interactions between sodium hypochlorite, chlorhexidine, EDTA, and citric acid. J Endod. 2012; 38:426–431. PMID: 22414823.28. Kato AS, Cunha RS, da Silveira Bueno CE, Pelegrine RA, Fontana CE, de Martin AS. Investigation of the efficacy of passive ultrasonic irrigation versus irrigation with reciprocating activation: an environmental scanning electron microscopic study. J Endod. 2016; 42:659–663. PMID: 26906240.29. Rödig T, Koberg C, Baxter S, Konietschke F, Wiegand A, Rizk M. Micro-CT evaluation of sonically and ultrasonically activated irrigation on the removal of hard-tissue debris from isthmus-containing mesial root canal systems of mandibular molars. Int Endod J. 2019; 52:1173–1181. PMID: 30773661.30. Rödig T, Döllmann S, Konietschke F, Drebenstedt S, Hülsmann M. Effectiveness of different irrigant agitation techniques on debris and smear layer removal in curved root canals: a scanning electron microscopy study. J Endod. 2010; 36:1983–1987. PMID: 21092817.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An evaluation of canal curvature at the apical one third in type II mesial canals of mandibular molars
- Time-dependent effects of EDTA application on removal of smear layer in the root canal system
- A sem observation on the efficiency preparation of oval canals using hand and engine-driven instruments
- Change of working length in curved canals by various instrumentation techniques
- In vitro evaluation of cleaning efficacy of various irrigation methods in mandibular molars