J Stroke.
2024 May;26(2):203-230. 10.5853/jos.2023.04329.
Neuroprotective Approaches for Brain Injury After Cardiac Arrest: Current Trends and Prospective Avenues
- Affiliations
-
- 1Department of Neurosurgery, University of Maryland School of Medicine, Baltimore, MD, USA
- 2Department of Orthopedics, University of Maryland School of Medicine, Baltimore, MD, USA
- 3Department of Anatomy and Neurobiology, University of Maryland School of Medicine, Baltimore, MD, USA
- 4Department of Biomedical Engineering, Johns Hopkins University School of Medicine, Baltimore, MD, USA
- KMID: 2556042
- DOI: http://doi.org/10.5853/jos.2023.04329
Abstract
- With the implementation of improved bystander cardiopulmonary resuscitation techniques and public-access defibrillation, survival after out-of-hospital cardiac arrest (OHCA) has increased significantly over the years. Nevertheless, OHCA survivors have residual anoxia/reperfusion brain damage and associated neurological impairment resulting in poor quality of life. Extracorporeal membrane oxygenation or targeted temperature management has proven effective in improving post-cardiac arrest (CA) neurological outcomes, yet considering the substantial healthcare costs and resources involved, there is an urgent need for alternative treatment strategies that are crucial to alleviate brain injury and promote recovery of neurological function after CA. In this review, we searched PubMed for the latest preclinical or clinical studies (2016–2023) utilizing gas-mediated, pharmacological, or stem cell-based neuroprotective approaches after CA. Preclinical studies utilizing various gases (nitric oxide, hydrogen, hydrogen sulfide, carbon monoxide, argon, and xenon), pharmacological agents targeting specific CA-related pathophysiology, and stem cells have shown promising results in rodent and porcine models of CA. Although inhaled gases and several pharmacological agents have entered clinical trials, most have failed to demonstrate therapeutic effects in CA patients. To date, stem cell therapies have not been reported in clinical trials for CA. A relatively small number of preclinical stem-cell studies with subtle therapeutic benefits and unelucidated mechanistic explanations warrant the need for further preclinical studies including the improvement of their therapeutic potential. The current state of the field is discussed and the exciting potential of stem-cell therapy to abate neurological dysfunction following CA is highlighted.
Figure
Reference
-
References
1. Kiguchi T, Okubo M, Nishiyama C, Maconochie I, Ong MEH, Kern KB, et al. Out-of-hospital cardiac arrest across the world: first report from the International Liaison Committee on Resuscitation (ILCOR). Resuscitation. 2020; 152:39–49.
Article2. Gräsner JT, Wnent J, Herlitz J, Perkins GD, Lefering R, Tjelmeland I, et al. Survival after out-of-hospital cardiac arrest in Europe - results of the EuReCa TWO study. Resuscitation. 2020; 148:218–226.
Article3. Gräsner JT, Herlitz J, Tjelmeland IBM, Wnent J, Masterson S, Lilja G, et al. European resuscitation council guidelines 2021: epidemiology of cardiac arrest in Europe. Resuscitation. 2021; 161:61–79.
Article4. Kragholm K, Wissenberg M, Mortensen RN, Hansen SM, Malta Hansen C, Thorsteinsson K, et al. Bystander efforts and 1-year outcomes in out-of-hospital cardiac arrest. N Engl J Med. 2017; 376:1737–1747.
Article5. Perkins GD, Callaway CW, Haywood K, Neumar RW, Lilja G, Rowland MJ, et al. Brain injury after cardiac arrest. Lancet. 2021; 398:1269–1278.
Article6. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019; 139:e56–e528.7. Hosseini M, Wilson RH, Crouzet C, Amirhekmat A, Wei KS, Akbari Y. Resuscitating the globally ischemic brain: TTM and beyond. Neurotherapeutics. 2020; 17:539–562.
Article8. Maekawa K, Tanno K, Hase M, Mori K, Asai Y. Extracorporeal cardiopulmonary resuscitation for patients with out-of-hospital cardiac arrest of cardiac origin: a propensity-matched study and predictor analysis. Crit Care Med. 2013; 41:1186–1196.
Article9. Sakurai T, Kaneko T, Yamada S, Takahashi T. Extracorporeal cardiopulmonary resuscitation with temperature management could improve the neurological outcomes of out-of-hospital cardiac arrest: a retrospective analysis of a nationwide multicenter observational study in Japan. J Intensive Care. 2022; 10:30.
Article10. Nolan JP, Sandroni C, Böttiger BW, Cariou A, Cronberg T, Friberg H, et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med. 2021; 47:369–421.
Article11. Panchal AR, Bartos JA, Cabañas JG, Donnino MW, Drennan IR, Hirsch KG, et al. Part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020; 142(16 suppl 2):S366–S468.12. Chen S, Lachance BB, Gao L, Jia X. Targeted temperature management and early neuro-prognostication after cardiac arrest. J Cereb Blood Flow Metab. 2021; 41:1193–1209.
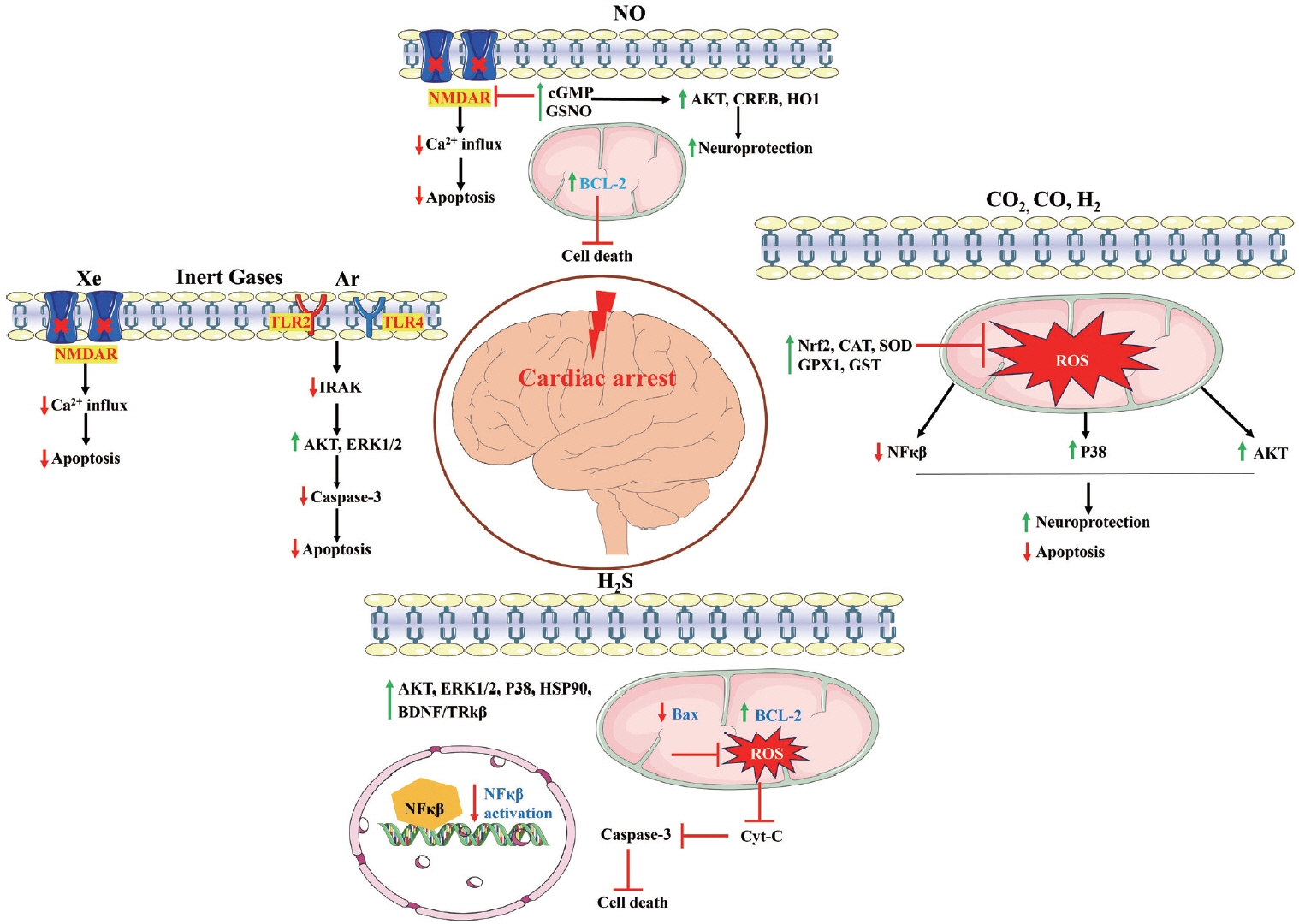
Article13. Hayashida K, Bagchi A, Miyazaki Y, Hirai S, Seth D, Silverman MG, et al. Improvement in outcomes after cardiac arrest and resuscitation by inhibition of S-nitrosoglutathione reductase. Circulation. 2019; 139:815–827.
Article14. Brücken A, Bleilevens C, Berger P, Nolte K, Gaisa NT, Rossaint R, et al. Effects of inhaled nitric oxide on outcome after prolonged cardiac arrest in mild therapeutic hypothermia treated rats. Sci Rep. 2018; 8:6743.
Article15. Dezfulian C, Kenny E, Lamade A, Misse A, Krehel N, St Croix C, et al. Mechanistic characterization of nitrite-mediated neuroprotection after experimental cardiac arrest. J Neurochem. 2016; 139:419–431.
Article16. Morgan RW, Sutton RM, Himebauch AS, Roberts AL, Landis WP, Lin Y, et al. A randomized and blinded trial of inhaled nitric oxide in a piglet model of pediatric cardiopulmonary resuscitation. Resuscitation. 2021; 162:274–283.17. Patel JK, Schoenfeld E, Hou W, Singer A, Rakowski E, Ahmad S, et al. Inhaled nitric oxide in adults with in-hospital cardiac arrest: a feasibility study. Nitric Oxide. 2021; 115:30–33.
Article18. Vitturi DA, Maynard C, Olsufka M, Straub AC, Krehel N, Kudenchuk PJ, et al. Nitrite elicits divergent NO-dependent signaling that associates with outcome in out of hospital cardiac arrest. Redox Biol. 2020; 32:101463.
Article19. Wang P, Yao L, Zhou LL, Liu YS, Chen MD, Wu HD, et al. Carbon monoxide improves neurologic outcomes by mitochondrial biogenesis after global cerebral ischemia induced by cardiac arrest in rats. Int J Biol Sci. 2016; 12:1000–1009.
Article20. Wu J, Li Y, Yang P, Huang Y, Lu S, Xu F. Novel role of carbon monoxide in improving neurological outcome after cardiac arrest in aged rats: involvement of inducing mitochondrial autophagy. J Am Heart Assoc. 2019; 8:e011851.
Article21. Wollborn J, Steiger C, Doostkam S, Schallner N, Schroeter N, Kari FA, et al. Carbon monoxide exerts functional neuroprotection after cardiac arrest using extracorporeal resuscitation in pigs. Crit Care Med. 2020; 48:e299–e307.22. Wang CH, Huang CH, Tsai MS, Wang CC, Chang WT, Liu SH, et al. Inhaled carbon dioxide improves neurological outcomes by downregulating hippocampal autophagy and apoptosis in an asphyxia-induced cardiac arrest and resuscitation rat model. J Am Heart Assoc. 2022; 11:e027685.
Article23. Babini G, Ristagno G, Boccardo A, De Giorgio D, De Maglie M, Affatato R, et al. Effect of mild hypercapnia on outcome and histological injury in a porcine post cardiac arrest model. Resuscitation. 2019; 135:110–117.
Article24. Chen G, Li J, Wang J, Chen B, Li Y. Inhaling hydrogen ameliorates early postresuscitation EEG characteristics in an asphyxial cardiac arrest rat model. Biomed Res Int. 2019; 2019:6410159.
Article25. Wang P, Jia L, Chen B, Zhang L, Liu J, Long J, et al. Hydrogen inhalation is superior to mild hypothermia in improving cardiac function and neurological outcome in an asphyxial cardiac arrest model of rats. Shock. 2016; 46:312–318.
Article26. Gao Y, Gui Q, Jin L, Yu P, Wu L, Cao L, et al. Hydrogen-rich saline attenuates hippocampus endoplasmic reticulum stress after cardiac arrest in rats. Neurosci Lett. 2017; 640:29–36.
Article27. Tamura T, Suzuki M, Hayashida K, Kobayashi Y, Yoshizawa J, Shibusawa T, et al. Hydrogen gas inhalation alleviates oxidative stress in patients with post-cardiac arrest syndrome. J Clin Biochem Nutr. 2020; 67:214–221.
Article28. Tamura T, Suzuki M, Homma K, Sano M; HYBRID II Study Group. Efficacy of inhaled hydrogen on neurological outcome following brain ischaemia during post-cardiac arrest care (HYBRID II): a multi-centre, randomised, double-blind, placebo-controlled trial. EClinicalMedicine. 2023; 58:101907.29. Koziakova M, Harris K, Edge CJ, Franks NP, White IL, Dickinson R. Noble gas neuroprotection: xenon and argon protect against hypoxic-ischaemic injury in rat hippocampus in vitro via distinct mechanisms. Br J Anaesth. 2019; 123:601–609.
Article30. Law LS, Lo EA, Chan CC, Gan TJ. Neurologic and cognitive outcomes associated with the clinical use of xenon: a systematic review and meta-analysis of randomized-controlled trials. Can J Anaesth. 2018; 65:1041–1056.
Article31. Zhao H, Mitchell S, Koumpa S, Cui YT, Lian Q, Hagberg H, et al. Heme oxygenase-1 mediates neuroprotection conferred by argon in combination with hypothermia in neonatal hypoxia-ischemia brain injury. Anesthesiology. 2016; 125:180–192.
Article32. Broad KD, Fierens I, Fleiss B, Rocha-Ferreira E, Ezzati M, Hassell J, et al. Inhaled 45-50% argon augments hypothermic brain protection in a piglet model of perinatal asphyxia. Neurobiol Dis. 2016; 87:29–38.
Article33. Fumagalli F, Olivari D, Boccardo A, De Giorgio D, Affatato R, Ceriani S, et al. Ventilation with argon improves survival with good neurological recovery after prolonged untreated cardiac arrest in pigs. J Am Heart Assoc. 2020; 9:e016494.
Article34. Laitio R, Hynninen M, Arola O, Virtanen S, Parkkola R, Saunavaara J, et al. Effect of inhaled xenon on cerebral white matter damage in comatose survivors of out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2016; 315:1120–1128.
Article35. Xin L, Junhua W, Long L, Jun Y, Yang X. Exogenous hydrogen sulfide protects SH-SY5Y cells from OGD/RInduced injury. Curr Mol Med. 2017; 17:563–567.
Article36. Li H, Zhu L, Feng J, Hu X, Li C, Zhang B. Hydrogen sulfide decreases blood-brain barrier damage via regulating protein kinase C and tight junction after cardiac arrest in rats. Cell Physiol Biochem. 2018; 47:994–1006.37. Sun X, Wang Y, Wen S, Huang K, Huang J, Chu X, et al. Novel controlled and targeted releasing hydrogen sulfide system exerts combinational cerebral and myocardial protection after cardiac arrest. J Nanobiotechnology. 2021; 19:40.
Article38. Lin J, Wu W, Xu Z, Liu S, Lu W, Pan M. Effects of NaHS and hydroxylamine on the expressions of brain-derived neurotrophic factor and its receptors in rats after cardiac arrest and cardiopulmonary resuscitation. Scand J Trauma Resusc Emerg Med. 2018; 26:109.
Article39. Huang L, Applegate RL 2nd, Applegate PM, Boling W, Zhang JH. Inhalation of high concentration hydrogen gas improves short-term outcomes in a rat model of asphyxia induced-cardiac arrest. Med Gas Res. 2018; 8:73–78.
Article40. Brücken A, Bleilevens C, Föhr P, Nolte K, Rossaint R, Marx G, et al. Influence of argon on temperature modulation and neurological outcome in hypothermia treated rats following cardiac arrest. Resuscitation. 2017; 117:32–39.
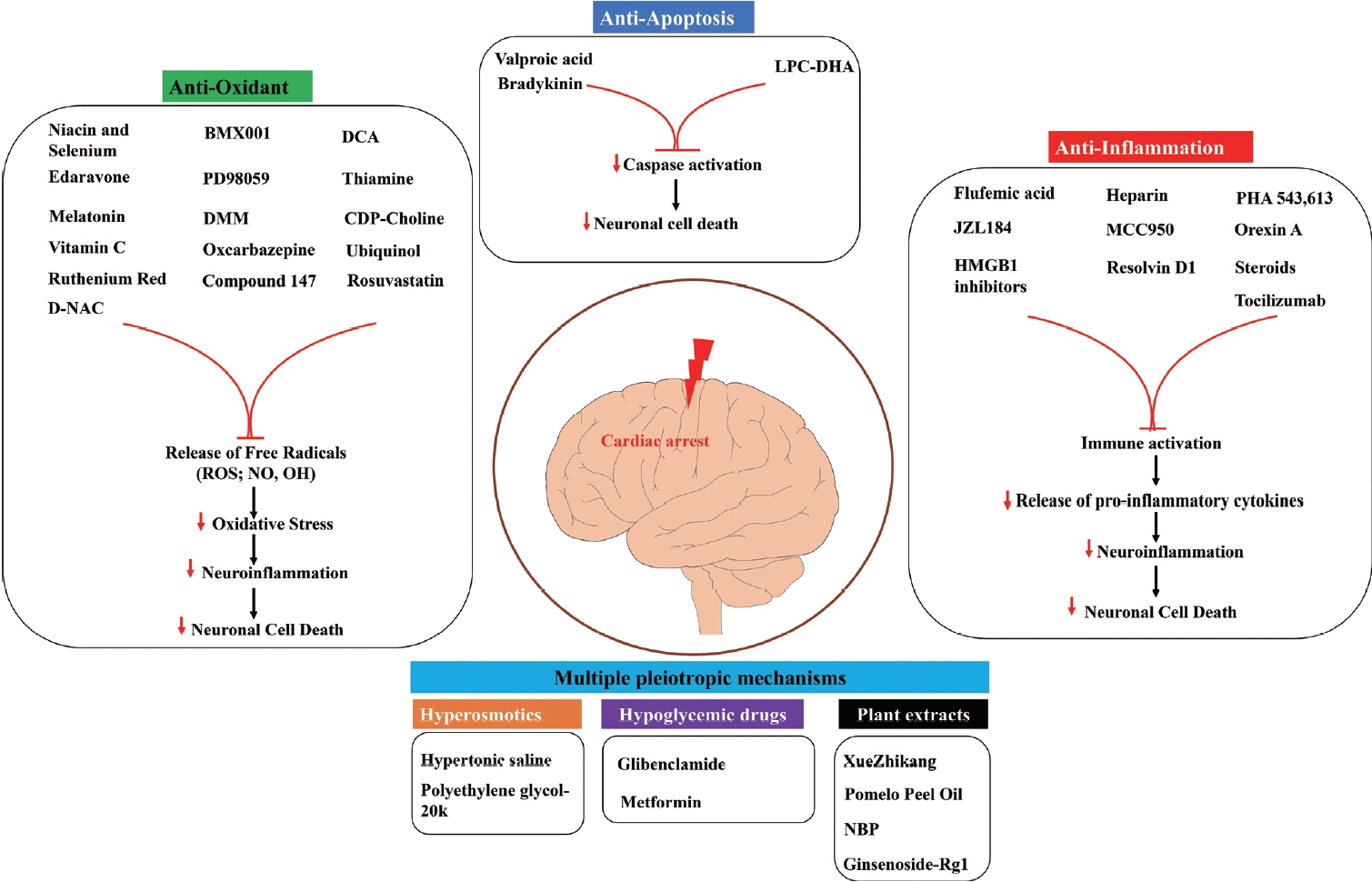
Article41. Kwon WY, Suh GJ, Kim KS, Jung YS, Kim SH, Lee AR, et al. Niacin and selenium attenuate brain injury after cardiac arrest in rats by up-regulating DJ-1-Akt signaling. Crit Care Med. 2018; 46:e788–e796.
Article42. Wang P, Li Y, Yan B, Yang Z, Li L, Cao Z, et al. Manganese porphyrin promotes post cardiac arrest recovery in mice and rats. Biology (Basel). 2022; 11:957.
Article43. Xu J, Pan H, Xie X, Zhang J, Wang Y, Yang G. Inhibiting succinate dehydrogenase by dimethyl malonate alleviates brain damage in a rat model of cardiac arrest. Neuroscience. 2018; 393:24–32.
Article44. Qiu Y, Wu Y, Meng M, Luo M, Zhao H, Sun H, et al. Rosuvastatin improves myocardial and neurological outcomes after asphyxial cardiac arrest and cardiopulmonary resuscitation in rats. Biomed Pharmacother. 2017; 87:503–508.
Article45. Qin T, Lei LY, Li N, Shi FR, Chen MH, Xie L. Edaravone improves survival and neurological outcomes after CPR in a ventricular fibrillation model of rats. Am J Emerg Med. 2016; 34:1944–1949.
Article46. Xiao Y, Su C, Zhang G, Liang L, Jin T, Bradley J, et al. Vitamin C improves the outcomes of cardiopulmonary resuscitation and alters shedding of syndecan-1 and p38/MAPK phosphorylation in a rat model. J Am Heart Assoc. 2022; 11:e023787.
Article47. Cho JH, Tae HJ, Kim IS, Song M, Kim H, Lee TK, et al. Melatonin alleviates asphyxial cardiac arrest-induced cerebellar Purkinje cell death by attenuation of oxidative stress. Exp Neurol. 2019; 320:112983.
Article48. Yang L, Wang J, Deng Y, Gong C, Li Q, Chen Q, et al. Melatonin improves neurological outcomes and preserves hippocampal mitochondrial function in a rat model of cardiac arrest. PLoS One. 2018; 13:e0207098.
Article49. He F, Zheng G, Hou J, Hu Q, Ling Q, Wu G, et al. N-acetylcysteine alleviates post-resuscitation myocardial dysfunction and improves survival outcomes via partly inhibiting NLRP3 inflammasome induced-pyroptosis. J Inflamm (Lond). 2020; 17:25.
Article50. Modi HR, Wang Q, Olmstead SJ, Khoury ES, Sah N, Guo Y, et al. Systemic administration of dendrimer N-acetyl cysteine improves outcomes and survival following cardiac arrest. Bioeng Transl Med. 2021; 7:e10259.
Article51. Medvedeva YV, Yin HZ, Bazrafkan A, Yeromin A, Ji SG, WeissHung EJ, et al. Blocking mitochondrial Zn2+ accumulation after ischemia reduces mitochondrial dysfunction and neuronal injury. J Neurosci. 2022; 42:5281–5292.
Article52. Salamah A, Mehrez M, Faheem A, El Amrousy D. Efficacy of citicoline as a neuroprotector in children with post cardiac arrest: a randomized controlled clinical trial. Eur J Pediatr. 2021; 180:1249–1255.
Article53. Cocchi MN, Giberson B, Berg K, Salciccioli JD, Naini A, Buettner C, et al. Coenzyme Q10 levels are low and associated with increased mortality in post-cardiac arrest patients. Resuscitation. 2012; 83:991–995.
Article54. Holmberg MJ, Andersen LW, Moskowitz A, Berg KM, Cocchi MN, Chase M, et al. Ubiquinol (reduced coenzyme Q10) as a metabolic resuscitator in post-cardiac arrest: a randomized, double-blind, placebo-controlled trial. Resuscitation. 2021; 162:388–395.
Article55. Nguyen Thi PA, Chen MH, Li N, Zhuo XJ, Xie L. PD98059 protects brain against cells death resulting from ROS/ERK activation in a cardiac arrest rat model. Oxid Med Cell Longev. 2016; 2016:3723762.56. Zheng JH, Xie L, Li N, Fu ZY, Tan XF, Tao R, et al. PD98059 protects the brain against mitochondrial-mediated apoptosis and autophagy in a cardiac arrest rat model. Life Sci. 2019; 232:116618.
Article57. Yuan ZL, Zhang ZX, Mo YZ, Li DL, Xie L, Chen MH. Inhibition of extracellular signal-regulated kinase downregulates endoplasmic reticulum stress-induced apoptosis and decreases brain injury in a cardiac arrest rat model. Physiol Res. 2022; 71:413–423.
Article58. Kim YH, Lee TK, Lee JC, Kim DW, Hong S, Cho JH, et al. Therapeutic administration of oxcarbazepine saves cerebellar purkinje cells from ischemia and reperfusion injury induced by cardiac arrest through attenuation of oxidative stress. Antioxidants (Basel). 2022; 11:2450.
Article59. Shen Y, Li R, Yu S, Zhao Q, Wang Z, Sheng H, et al. Activation of the ATF6 (activating transcription factor 6) signaling pathway in neurons improves outcome after cardiac arrest in mice. J Am Heart Assoc. 2021; 10:e020216.
Article60. Yuan Z, Lu L, Lian Y, Zhao Y, Tang T, Xu S, et al. AA147 ameliorates post-cardiac arrest cerebral ischemia/reperfusion injury through the co-regulation of the ATF6 and Nrf2 signaling pathways. Front Pharmacol. 2022; 13:1028002.
Article61. Ikeda K, Liu X, Kida K, Marutani E, Hirai S, Sakaguchi M, et al. Thiamine as a neuroprotective agent after cardiac arrest. Resuscitation. 2016; 105:138–144.
Article62. Wang P, Chen M, Yang Z, Yu T, Zhu J, Zhou L, et al. Activation of pyruvate dehydrogenase activity by dichloroacetate improves survival and neurologic outcomes after cardiac arrest in rats. Shock. 2018; 49:704–711.
Article63. Nishikimi M, Yagi T, Shoaib M, Takegawa R, Rasul R, Hayashida K, et al. Phospholipid screening postcardiac arrest detects decreased plasma lysophosphatidylcholine: supplementation as a new therapeutic approach. Crit Care Med. 2022; 50:e199–e208.
Article64. Nishikimi M, Shoaib M, Choudhary RC, Aoki T, Miyara SJ, Yagi T, et al. Preserving brain LPC-DHA by plasma supplementation attenuates brain injury after cardiac arrest. Ann Neurol. 2022; 91:389–403.
Article65. Lin SR, Lin QM, Lin YJ, Qian X, Wang XP, Gong Z, et al. Bradykinin postconditioning protects rat hippocampal neurons after restoration of spontaneous circulation following cardiac arrest via activation of the AMPK/mTOR signaling pathway. Neural Regen Res. 2022; 17:2232–2237.
Article66. Oh JS, Tulasi J, Xiaodan R, Stacey WC, Neumar RW. Valproic acid combined with postcardiac arrest hypothermic-targeted temperature management prevents delayed seizures and improves survival in a rat cardiac arrest model. Crit Care Med. 2017; 45:e1149–e1156.
Article67. Oh JS, Park J, Kim K, Jeong HH, Oh YM, Choi S, et al. HSP70-mediated neuroprotection by combined treatment of valproic acid with hypothermia in a rat asphyxial cardiac arrest model. PLoS One. 2021; 16:e0253328.
Article68. Zhou X, Liu Y, Huang Y, Zhu S, Zhu J, Wang R. Hypertonic saline infusion suppresses apoptosis of hippocampal cells in a rat model of cardiopulmonary resuscitation. Sci Rep. 2017; 7:5783.
Article69. Liu W, Ye Q, Xi W, Li Y, Zhou X, Wang Y, et al. The ERK/CREB/PTN/syndecan-3 pathway involves in heparin-mediated neuro-protection and neuro-regeneration against cerebral ischemia-reperfusion injury following cardiac arrest. Int Immunopharmacol. 2021; 98:107689.
Article70. Chen J, Chang Y, Zhu J, Peng Y, Li Z, Zhang K, et al. Flufenamic acid improves survival and neurologic outcome after successful cardiopulmonary resuscitation in mice. J Neuroinflammation. 2022; 19:214.
Article71. Tsai MS, Chuang PY, Yu PH, Huang CH, Tang CH, Chang WT, et al. Glucocorticoid use during cardiopulmonary resuscitation may be beneficial for cardiac arrest. Int J Cardiol. 2016; 222:629–635.72. Tsai MS, Chuang PY, Huang CH, Tang CH, Yu PH, Chang WT, et al. Postarrest steroid use may improve outcomes of cardiac arrest survivors. Crit Care Med. 2019; 47:167–175.
Article73. Chen W, Wang H, Wang Z, Zhao C, Xu J, Chen Q. Resolvin D1 improves post-resuscitation cardiac and cerebral outcomes in a porcine model of cardiac arrest. Shock. 2020; 54:548–554.
Article74. Han R, Zhang G, Qiao X, Guo Y, Sun L, Li J, et al. α7 nicotinic acetylcholine receptor mediates the neuroprotection of remote ischemic postconditioning in a rat model of asphyxial cardiac arrest. J Surg Res. 2020; 246:6–18.
Article75. Xu J, Zheng G, Hu J, Ge W, Bradley JL, Ornato JP, et al. The monoacylglycerol lipase inhibitor, JZL184, has comparable effects to therapeutic hypothermia, attenuating global cerebral injury in a rat model of cardiac arrest. Biomed Pharmacother. 2022; 156:113847.
Article76. Koenig MA, Jia X, Kang X, Velasquez A, Thakor NV, Geocadin RG. Intraventricular orexin-A improves arousal and early EEG entropy in rats after cardiac arrest. Brain Res. 2009; 1255:153–161.
Article77. Kang YJ, Tian G, Bazrafkan A, Farahabadi MH, Azadian M, Abbasi H, et al. Recovery from coma post-cardiac arrest is dependent on the orexin pathway. J Neurotrauma. 2017; 34:2823–2832.
Article78. Modi HR, Wang Q, Gd S, Sherman D, Greenwald E, Savonenko AV, et al. Intranasal post-cardiac arrest treatment with orexin-A facilitates arousal from coma and ameliorates neuroinflammation. PLoS One. 2017; 12:e0182707.
Article79. Shi X, Li M, Huang K, Zhou S, Hu Y, Pan S, et al. HMGB1 binding heptamer peptide improves survival and ameliorates brain injury in rats after cardiac arrest and cardiopulmonary resuscitation. Neuroscience. 2017; 360:128–138.
Article80. Boissady E, Abi Zeid Daou Y, Faucher E, Kohlhauer M, Lidouren F, El Hedjaj C, et al. High-mobility group box 1-signaling inhibition with glycyrrhizin prevents cerebral T-cell infiltration after cardiac arrest. J Am Heart Assoc. 2023; 12:e027749.
Article81. Jiang M, Li R, Lyu J, Li X, Wang W, Wang Z, et al. MCC950, a selective NLPR3 inflammasome inhibitor, improves neurologic function and survival after cardiac arrest and resuscitation. J Neuroinflammation. 2020; 17:256.
Article82. Chang Y, Zhu J, Wang D, Li H, He Y, Liu K, et al. NLRP3 inflammasome-mediated microglial pyroptosis is critically involved in the development of post-cardiac arrest brain injury. J Neuroinflammation. 2020; 17:219.
Article83. Zheng G, Xu J, He F, Hu J, Ge W, Ji X, et al. Effects of NLRP3 inflammasome blockade on postresuscitation cerebral function in a rat model of cardiopulmonary resuscitation. Biomed Pharmacother. 2021; 143:112093.
Article84. Yang J, Xiao Y, Quan EY, Hu Z, Guo Q, Miao C, et al. Effects of polyethylene glycol-20k on postresuscitation myocardial and cerebral function in a rat model of cardiopulmonary resuscitation. Crit Care Med. 2018; 46:e1190–e1195.
Article85. Ge W, Zheng G, Ji X, He F, Hu J, Bradley JL, et al. Effects of polyethylene glycol-20k on coronary perfusion pressure and postresuscitation myocardial and cerebral function in a rat model of cardiac arrest. J Am Heart Assoc. 2020; 9:e014232.
Article86. Guo Q, Yang J, Hu Z, Xiao Y, Wu X, Bradley J, et al. Polyethylene glycol-20k reduces post-resuscitation cerebral dysfunction in a rat model of cardiac arrest and resuscitation: a potential mechanism. Biomed Pharmacother. 2021; 139:111646.
Article87. Nakayama S, Migliati E, Amiry-Moghaddam M, Ottersen OP, Bhardwaj A. Osmotherapy with hypertonic saline attenuates global cerebral edema following experimental cardiac arrest via perivascular pool of aquaporin-4. Crit Care Med. 2016; 44:e702–e710.
Article88. Zeng J, Zhu L, Liu J, Zhu T, Xie Z, Sun X, et al. Metformin protects against oxidative stress injury induced by ischemia/reperfusion via regulation of the lncRNA-H19/miR-148a-3p/ Rock2 axis. Oxid Med Cell Longev. 2019; 2019:8768327.89. Shoaib M, Choudhary RC, Chillale RK, Kim N, Miyara SJ, Haque S, et al. Metformin-mediated mitochondrial protection post-cardiac arrest improves EEG activity and confers neuroprotection and survival benefit. FASEB J. 2022; 36:e22307.
Article90. Chuan L, Huang X, Fan C, Wen S, Yang X, Wang J, et al. Metformin ameliorates brain damage caused by cardiopulmonary resuscitation via targeting endoplasmic reticulum stress-related proteins GRP78 and XBP1. Eur J Pharmacol. 2021; 891:173716.
Article91. Zhu J, Liu K, Huang K, Gu Y, Hu Y, Pan S, et al. Metformin improves neurologic outcome via AMP-activated protein kinase–mediated autophagy activation in a rat model of cardiac arrest and resuscitation. J Am Heart Assoc. 2018; 7:e008389.92. Huang K, Wang Z, Gu Y, Hu Y, Ji Z, Wang S, et al. Glibenclamide is comparable to target temperature management in improving survival and neurological outcome after asphyxial cardiac arrest in rats. J Am Heart Assoc. 2016; 5:e003465.
Article93. Huang K, Wang Z, Gu Y, Ji Z, Lin Z, Wang S, et al. Glibenclamide prevents water diffusion abnormality in the brain after cardiac arrest in rats. Neurocrit Care. 2018; 29:128–135.
Article94. Lachance BB, Wang Z, Badjatia N, Jia X. The effect of glibenclamide on somatosensory evoked potentials after cardiac arrest in rats. Neurocrit Care. 2022; 36:612–620.
Article95. Wang Z, Zhang S, Du J, Lachance BB, Chen S, Polster BM, et al. Neuroprotection of NSC therapy is superior to glibenclamide in cardiac arrest-induced brain injury via neuroinflammation regulation. Transl Stroke Res. 2023; 14:723–739.
Article96. He Y, Chang Y, Peng Y, Zhu J, Liu K, Chen J, et al. Glibenclamide directly prevents neuroinflammation by targeting SUR1-TRPM4-mediated NLRP3 inflammasome activation in microglia. Mol Neurobiol. 2022; 59:6590–6607.
Article97. Liang L, Shao W, Shu T, Zhang Y, Xu S, Guo L, et al. Xuezhikang improves the outcomes of cardiopulmonary resuscitation in rats by suppressing the inflammation response through TLR4/NF-κB pathway. Biomed Pharmacother. 2019; 114:108817.
Article98. Yang S, Yu C, Yang Z, Cui H, Wu Y, Liang Z, et al. DL-3-n-butylphthalide-induced neuroprotection in rat models of asphyxia-induced cardiac arrest followed by cardiopulmonary resuscitation. J Cell Physiol. 2021; 236:7464–7472.
Article99. Wang W, Xie L, Zou X, Hu W, Tian X, Zhao G, et al. Pomelo peel oil suppresses TNF-α-induced necroptosis and cerebral ischaemia-reperfusion injury in a rat model of cardiac arrest. Pharm Biol. 2021; 59:401–409.
Article100. Wu Z, Huang J, Bai X, Wang Q, Wang F, Xu J, et al. Ginsenoside-Rg1 mitigates cardiac arrest-induced cognitive damage by modulating neuroinflammation and hippocampal plasticity. Eur J Pharmacol. 2023; 938:175431.
Article101. Perkins GD, Ji C, Deakin CD, Quinn T, Nolan JP, Scomparin C, et al. A randomized trial of epinephrine in out-of-hospital cardiac arrest. N Engl J Med. 2018; 379:711–721.
Article102. Vammen L, Johannsen CM, Baltsen CD, Nørholt C, Eggertsen M, Mortensen S, et al. Thiamine for the treatment of cardiac arrest-induced neurological injury: a randomized, blinded, placebo-controlled experimental study. J Am Heart Assoc. 2023; 12:e028558.
Article103. Pradita-Ukrit S, Vattanavanit V. Efficacy of thiamine in the treatment of postcardiac arrest patients: a randomized controlled study. Crit Care Res Pract. 2020; 2020:2981079.
Article104. Andersen LW, Isbye D, Kjærgaard J, Kristensen CM, Darling S, Zwisler ST, et al. Effect of vasopressin and methylprednisolone vs placebo on return of spontaneous circulation in patients with in-hospital cardiac arrest: a randomized clinical trial. JAMA. 2021; 326:1586–1594.
Article105. Meyer MAS, Wiberg S, Grand J, Meyer ASP, Obling LER, Frydland M, et al. Treatment effects of interleukin-6 receptor antibodies for modulating the systemic inflammatory response after out-of-hospital cardiac arrest (the IMICA trial): a double-blinded, placebo-controlled, single-center, randomized, clinical trial. Circulation. 2021; 143:1841–1851.
Article106. Vallentin MF, Granfeldt A, Meilandt C, Povlsen AL, Sindberg B, Holmberg MJ, et al. Effect of intravenous or intraosseous calcium vs saline on return of spontaneous circulation in adults with out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2021; 326:2268–2276.
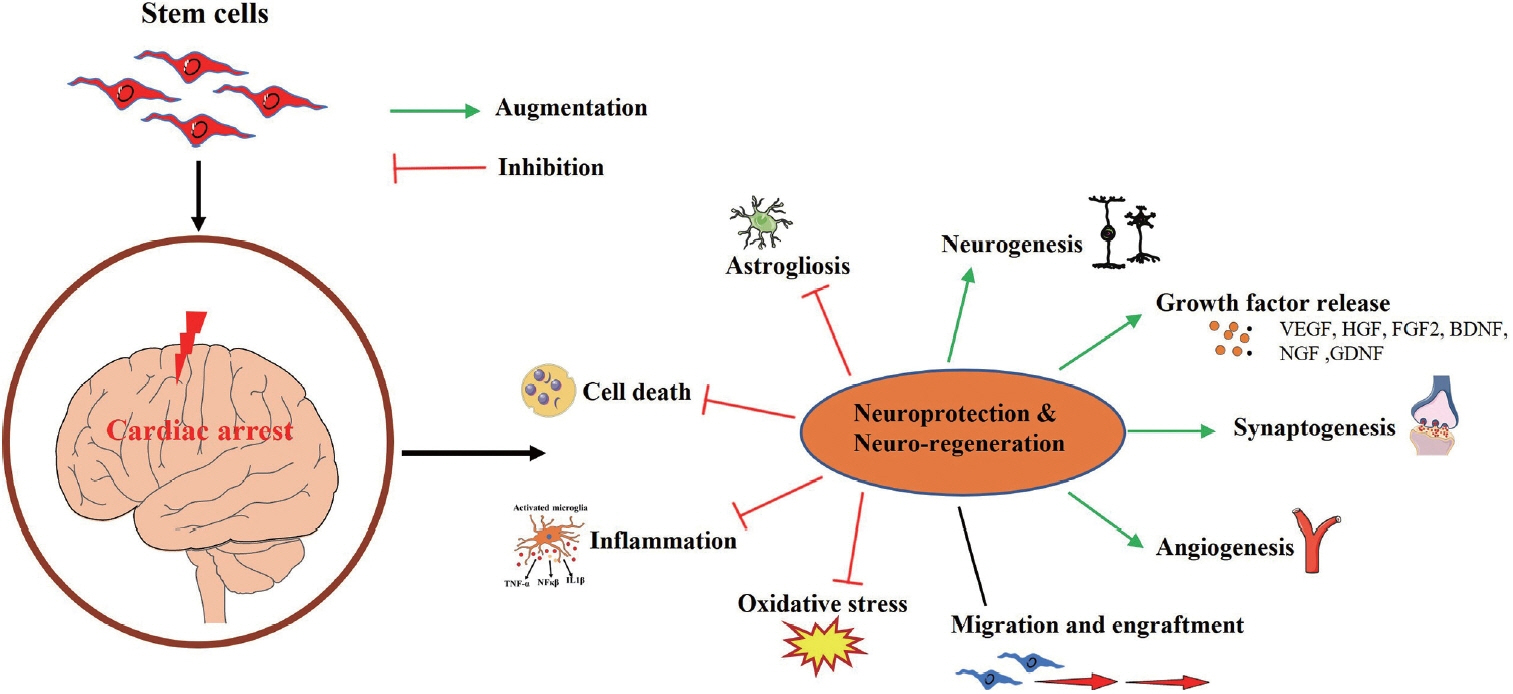
Article107. Tang X, Chen F, Lin Q, You Y, Ke J, Zhao S. Bone marrow mesenchymal stem cells repair the hippocampal neurons and increase the expression of IGF-1 after cardiac arrest in rats. Exp Ther Med. 2017; 14:4312–4320.
Article108. Lin QM, Tang XH, Lin SR, Chen BD, Chen F. Bone marrow-derived mesenchymal stem cell transplantation attenuates overexpression of inflammatory mediators in rat brain after cardiopulmonary resuscitation. Neural Regen Res. 2020; 15:324–331.
Article109. Yu Y, Wang D, Li H, Fan J, Liu Y, Zhao X, et al. Mesenchymal stem cells derived from induced pluripotent stem cells play a key role in immunomodulation during cardiopulmonary resuscitation. Brain Res. 2019; 1720:146293.
Article110. Xu J, Zhang M, Liu F, Shi L, Jiang X, Chen C, et al. Mesenchymal stem cells alleviate post-resuscitation cardiac and cerebral injuries by inhibiting cell pyroptosis and ferroptosis in a swine model of cardiac arrest. Front Pharmacol. 2021; 12:793829.
Article111. Zhou L, Lin Q, Wang P, Yao L, Leong K, Tan Z, et al. Enhanced neuroprotective efficacy of bone marrow mesenchymal stem cells co-overexpressing BDNF and VEGF in a rat model of cardiac arrest-induced global cerebral ischemia. Cell Death Dis. 2017; 8:e2774.
Article112. Wang Z, Du J, Lachance BB, Mascarenhas C, He J, Jia X. Intracerebroventricular administration of hNSCs improves neurological recovery after cardiac arrest in rats. Stem Cell Rev Rep. 2021; 17:923–937.
Article113. Meyer P, Grandgirard D, Lehner M, Haenggi M, Leib SL. Grafted neural progenitor cells persist in the injured site and differentiate neuronally in a rodent model of cardiac arrest-induced global brain ischemia. Stem Cells Dev. 2020; 29:574–585.
Article114. Li F, Zhang J, Chen A, Liao R, Duan Y, Xu Y, et al. Combined transplantation of neural stem cells and bone marrow mesenchymal stem cells promotes neuronal cell survival to alleviate brain damage after cardiac arrest via microRNA-133b incorporated in extracellular vesicles. Aging (Albany NY). 2021; 13:262–278.
Article115. Muir KW, Bulters D, Willmot M, Sprigg N, Dixit A, Ward N, et al. Intracerebral implantation of human neural stem cells and motor recovery after stroke: multicentre prospective single-arm study (PISCES-2). J Neurol Neurosurg Psychiatry. 2020; 91:396–401.
Article116. Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016; 388:787–796.117. Zhang S, Lachance BB, Moiz B, Jia X. Optimizing stem cell therapy after ischemic brain injury. J Stroke. 2020; 22:286–305.
Article118. Du J, Agatemor C, Saeui CT, Bhattacharya R, Jia X, Yarema KJ. Glycoengineering human neural and adipose stem cells with novel thiol-modified N-acetylmannosamine (ManNAc) analogs. Cells. 2021; 10:377.
Article119. Du J, Liu X, Yarema KJ, Jia X. Glycoengineering human neural stem cells (hNSCs) for adhesion improvement using a novel thiol-modified N-acetylmannosamine (ManNAc) analog. Biomater Adv. 2022; 134:112675.
Article120. Du J, Liu X, Marasini S, Wang Z, Dammen-Brower K, Yarema KJ, et al. Metabolically glycoengineered neural stem cells boost neural repair after cardiac arrest. Adv Funct Mater. 2023; 34:2309866.
Article121. Liu X, Jia X. Neuroprotection of stem cells against ischemic brain injury: from bench to clinic. Transl Stroke Res. 2023 Jul 7 [Epub]. https://doi.org/10.1007/s12975-023-01163-3.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of Brain Injury after Post-cardiac Arrest Syndrome
- Effects of N6-L-phenylisopropyl Adenosine in Rats after Asphyxial Cardiac Arrest
- Targeted temperature management with hypothermia for comatose patients after cardiac arrest
- Complete Recovery of Perfusion Abnormalities in a Cardiac Arrest Patient Treated with Hypothermia: Results of Cerebral Perfusion MR Imaging
- Clinical Implementation of Therapeutic Hypothermia after Cardiac Arrest