J Stroke.
2024 May;26(2):179-189. 10.5853/jos.2023.04287.
Prevalence of Cerebral Amyloid Angiopathy Pathology and Strictly Lobar Microbleeds in East-Asian Versus Western Populations: A Systematic Review and Meta-Analysis
- Affiliations
-
- 1Department of Neurology, Radboud University Medical Center, Donders Institute for Brain, Cognition and Behaviour, Nijmegen, The Netherlands
- 2Radboud Alzheimer Centre, Radboud University Medical Center, Nijmegen, The Netherlands
- 3Department of Genetics, Radboud University Medical Center, Nijmegen, The Netherlands
- KMID: 2556040
- DOI: http://doi.org/10.5853/jos.2023.04287
Abstract
- Background and Purpose
Possible differences in the prevalence of cerebral amyloid angiopathy (CAA) in East-Asian compared to Western populations have received little attention, and results so far have been ambiguous. Our aim is to compare the prevalence of CAA neuropathology and magnetic resonance imaging markers of CAA in East-Asian and Western cohorts reflecting the general population, cognitively normal elderly, patients with Alzheimer’s disease (AD), and patients with (lobar) intracerebral hemorrhage (ICH).
Methods
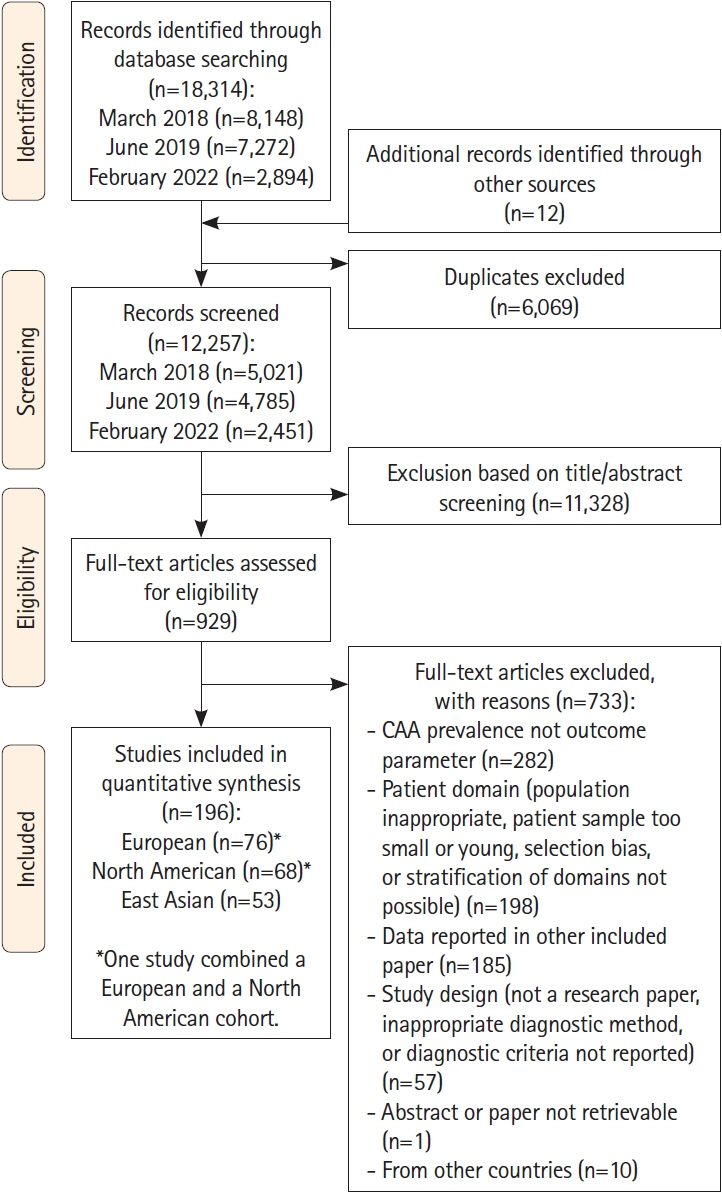
We performed a systematic literature search in PubMed and Embase for original research papers on the prevalence of CAA and imaging markers of CAA published up until February 17th 2022. Records were screened by two independent reviewers. Pooled estimates were determined using random-effects models. We compared studies from Japan, China, Taiwan, South Korea (East-Asian cohorts) to studies from Europe or North America (Western cohorts) by meta-regression models.
Results
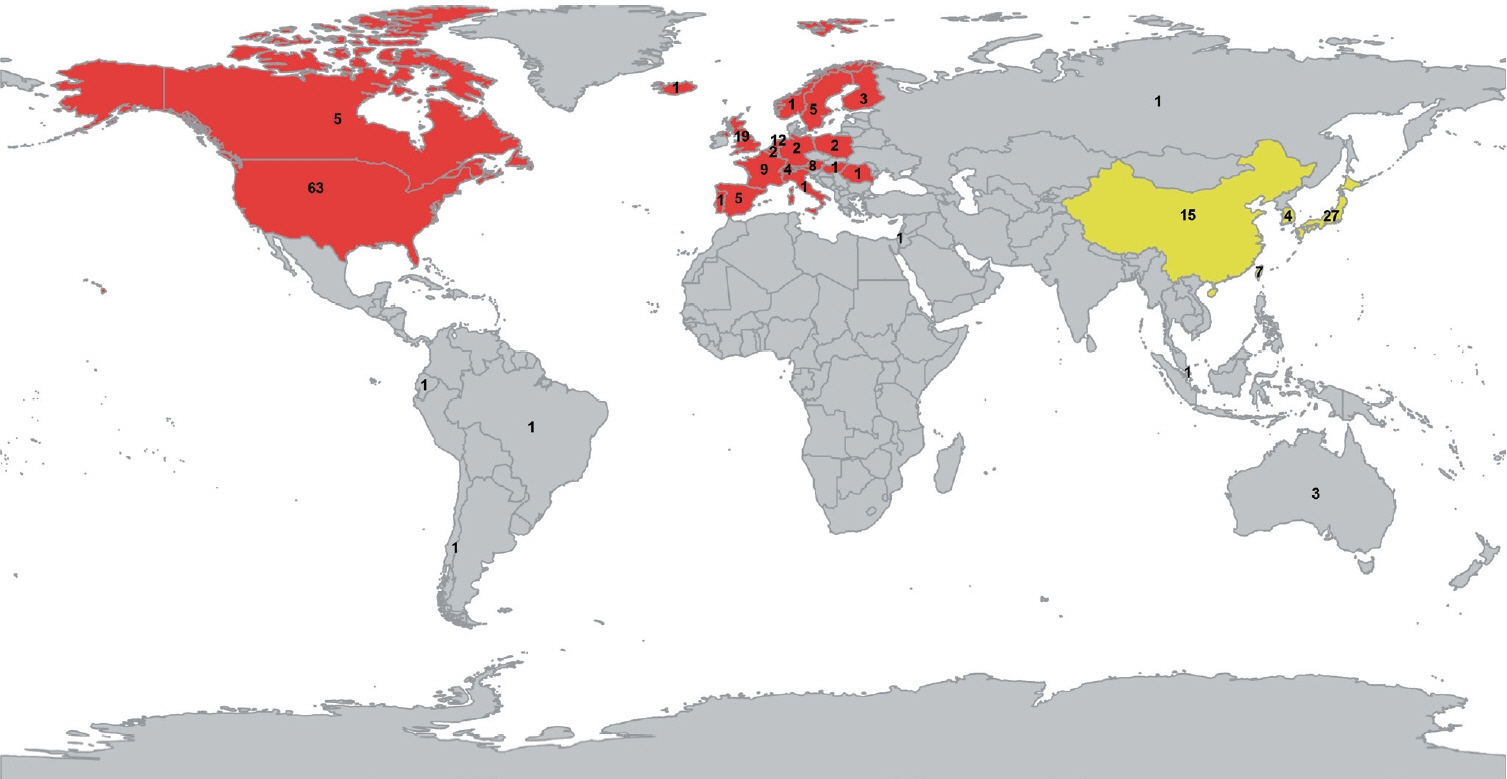
We identified 12,257 unique records, and we included 143 studies on Western study populations and 53 studies on East-Asian study populations. Prevalence of CAA neuropathology did not differ between East-Asian and Western cohorts in any of the investigated patient domains. The prevalence of strictly lobar microbleeds was lower in East-Asian cohorts of population-based individuals (5.6% vs. 11.4%, P=0.020), cognitively normal elderly (2.6% vs. 11.4%, P=0.001), and patients with ICH (10.2% vs. 24.6%, P<0.0001). However, age was in general lower in the East-Asian cohorts.
Conclusion
The prevalence of CAA neuropathology in the general population, cognitively normal elderly, patients with AD, and patients with (lobar) ICH is similar in East-Asian and Western countries. In East-Asian cohorts reflecting the general population, cognitively normal elderly, and patients with ICH, strictly lobar microbleeds were less prevalent, likely due to their younger age. Consideration of potential presence of CAA is warranted in decisions regarding antithrombotic treatment and potential new anti-amyloid-β immunotherapy as treatment for AD in East-Asian and Western countries alike.
Keyword
Figure
Reference
-
References
1. Attems J, Jellinger K, Thal DR, Van Nostrand W. Review: sporadic cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2011; 37:75–93.
Article2. Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ. Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways. Nat Rev Neurol. 2020; 16:30–42.3. Jäkel L, De Kort AM, Klijn CJM, Schreuder FHBM, Verbeek MM. Prevalence of cerebral amyloid angiopathy: a systematic review and meta-analysis. Alzheimers Dement. 2022; 18:10–28.
Article4. Smith EE, Charidimou A, Ayata C, Werring DJ, Greenberg SM. Cerebral amyloid angiopathy-related transient focal neurologic episodes. Neurology. 2021; 97:231–238.
Article5. Tanaka T, Fukuma K, Abe S, Matsubara S, Ikeda S, Kamogawa N, et al. Association of cortical superficial siderosis with poststroke epilepsy. Ann Neurol. 2023; 93:357–370.
Article6. Wermer MJH, Greenberg SM. The growing clinical spectrum of cerebral amyloid angiopathy. Curr Opin Neurol. 2018; 31:28–35.
Article7. Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, et al. Consequence of Aβ immunization on the vasculature of human Alzheimer’s disease brain. Brain. 2008; 131(Pt 12):3299–3310.8. Budd Haeberlein S, Aisen PS, Barkhof F, Chalkias S, Chen T, Cohen S, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022; 9:197–210.
Article9. van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023; 388:9–21.
Article10. Charidimou A, Boulouis G, Frosch MP, Baron JC, Pasi M, Albucher JF, et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 2022; 21:714–725.11. Sveikata L, Charidimou A, Viswanathan A. Vessels sing their ARIAs: the role of vascular amyloid in the age of aducanumab. Stroke. 2022; 53:298–302.
Article12. Yakushiji Y, Tanaka J, Wilson D, Charidimou A, Noguchi T, Kawashima M, et al. Proportion of intracerebral haemorrhage due to cerebral amyloid angiopathy in the East and West: comparison between single hospital centres in Japan and the United Kingdom. J Neurol Sci. 2020; 416:117037.13. Ng TH, Leung SY, Wong MP. Cerebral amyloid angiopathy in Chinese: incidence and significance. Clin Neurol Neurosurg. 1991; 93:19–23.
Article14. Masuda J, Tanaka K, Ueda K, Omae T. Autopsy study of incidence and distribution of cerebral amyloid angiopathy in Hisayama, Japan. Stroke. 1988; 19:205–210.
Article15. Chen YW, Lee MJ, Smith EE. Cerebral amyloid angiopathy in East and West. Int J Stroke. 2010; 5:403–411.
Article16. Yakushiji Y, Wilson D, Ambler G, Charidimou A, Beiser A, van Buchem MA, et al. Distribution of cerebral microbleeds in the East and West: individual participant meta-analysis. Neurology. 2019; 92:e1086–e1097.17. Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010; 74:1346–1350.18. Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001; 56:537–539.
Article19. Veritas Health Innovation. Covidence systematic review software [Internet]. Melbourne: Veritas Health Innovation; 2022 [accessed February 1, 2022]. Available from: www.covidence.org.20. Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012; 65:934–939.
Article21. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis [Internet]. Ottawa: Ottawa Hospital Research Institute; 2017 [accessed May 26, 2023]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.22. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–1558.
Article23. Shoamanesh A, Akoudad S, Himali JJ, Beiser AS, DeCarli C, Seshadri S, et al. Cortical superficial siderosis in the general population: the Framingham heart and Rotterdam studies. Int J Stroke. 2021; 16:798–808.
Article24. Eisai. “LEQEMBI® intravenous infusion” (Lecanemab) approved for the treatment of Alzheimer’s disease in Japan [Internet]. Eisai: Tokyo; 2023 [accessed March 1, 2024]. Available from: https://www.eisai.com/news/2023/news202359.html.25. Biogen. “LEQEMBI®” (Lecanemab) approved for the treatment of Alzheimer’s disease in China [Internet]. Cambridge: Biogen; 2024 [accessed March 1, 2024]. Available from: https://investors.biogen.com/news-releases/news-releasedetails/leqembir-lecanemab-approved-treatment-alzheimers-disease-china.26. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
Article27. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023; 613:227–228.
Article28. Schneider LS. Aducanumab trials EMERGE but don’t ENGAGE. J Prev Alzheimers Dis. 2022; 9:193–196.
Article29. Salloway S, Chalkias S, Barkhof F, Burkett P, Barakos J, Purcell D, et al. Amyloid-related imaging abnormalities in 2 phase 3 studies evaluating aducanumab in patients with early Alzheimer disease. JAMA Neurol. 2022; 79:13–21.
Article30. Yu L, Boyle PA, Nag S, Leurgans S, Buchman AS, Wilson RS, et al. APOE and cerebral amyloid angiopathy in community-dwelling older persons. Neurobiol Aging. 2015; 36:2946–2953.
Article31. Alzforum. Unlocking blood-brain barrier boosts immunotherapy efficacy, lowers ARIA [Internet]. Boston: Alzforum; 2023 [accessed March 1, 2024]. Available from: https://www.alzforum.org/news/conference-coverage/unlocking-blood-brainbarrier-boosts-immunotherapy-efficacy-lowers-aria#tying.32. Rodrigues MA, Samarasekera N, Lerpiniere C, Humphreys C, McCarron MO, White PM, et al. The Edinburgh CT and genetic diagnostic criteria for lobar intracerebral haemorrhage associated with cerebral amyloid angiopathy: model development and diagnostic test accuracy study. Lancet Neurol. 2018; 17:232–240.33. van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010; 9:167–176.
Article34. Jolink WMT, Wiegertjes K, Rinkel GJE, Algra A, de Leeuw FE, Klijn CJM. Location-specific risk factors for intracerebral hemorrhage: systematic review and meta-analysis. Neurology. 2020; 95:e1807–e1818.35. Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010; 41(10 Suppl):S103–S106.36. Young JH, Chang YP, Kim JD, Chretien JP, Klag MJ, Levine MA, et al. Differential susceptibility to hypertension is due to selection during the out-of-Africa expansion. PLoS Genet. 2005; 1:e82.
Article37. Tzourio C, Arima H, Harrap S, Anderson C, Godin O, Woodward M, et al. APOE genotype, ethnicity, and the risk of cerebral hemorrhage. Neurology. 2008; 70:1322–1328.38. Zhao D, Zhang Z, Wu GB, Wang HY, Gao F, Duan XD, et al. Apolipoprotein E gene polymorphism and the risk of subarachnoid hemorrhage: a meta-analysis of case-control studies. Acta Neurochir (Wien). 2016; 158:1515–1522.
Article39. Rutten JW, Dauwerse HG, Gravesteijn G, van Belzen MJ, van der Grond J, Polke JM, et al. Archetypal NOTCH3 mutations frequent in public exome: implications for CADASIL. Ann Clin Transl Neurol. 2016; 3:844–853.
Article40. Lai QL, Zhang YX, Wang JJ, Mo YJ, Zhuang LY, Cheng L, et al. Occurrence of intracranial hemorrhage and associated risk factors in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: a systematic review and meta-analysis. J Clin Neurol. 2022; 18:499–506.
Article41. Sveinbjornsdottir S, Sigurdsson S, Aspelund T, Kjartansson O, Eiriksdottir G, Valtysdottir B, et al. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry. 2008; 79:1002–1006.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lobar Intracerebral Hemorrhage Associated With Cortical Superficial Siderosis
- Cerebral Amyloid Angiopathy: A Systematic Review
- Characteristics of Cerebral Microbleeds
- Distribution of Cerebral Microbleeds Determines Their Association with Impaired Kidney Function
- Cerebral Microbleeds: Their Associated Factors, Radiologic Findings, and Clinical Implications