J Stroke.
2024 May;26(2):164-178. 10.5853/jos.2023.03279.
Cancer-Associated Stroke: Thrombosis Mechanism, Diagnosis, Outcome, and Therapeutic Strategies
- Affiliations
-
- 1Department of Neurology, Yonsei University College of Medicine, Seoul, Korea
- 2Integrative Research Center for Cerebrovascular and Cardiovascular Diseases, Seoul, Korea
- 3Department of Neurology, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea
- KMID: 2556039
- DOI: http://doi.org/10.5853/jos.2023.03279
Abstract
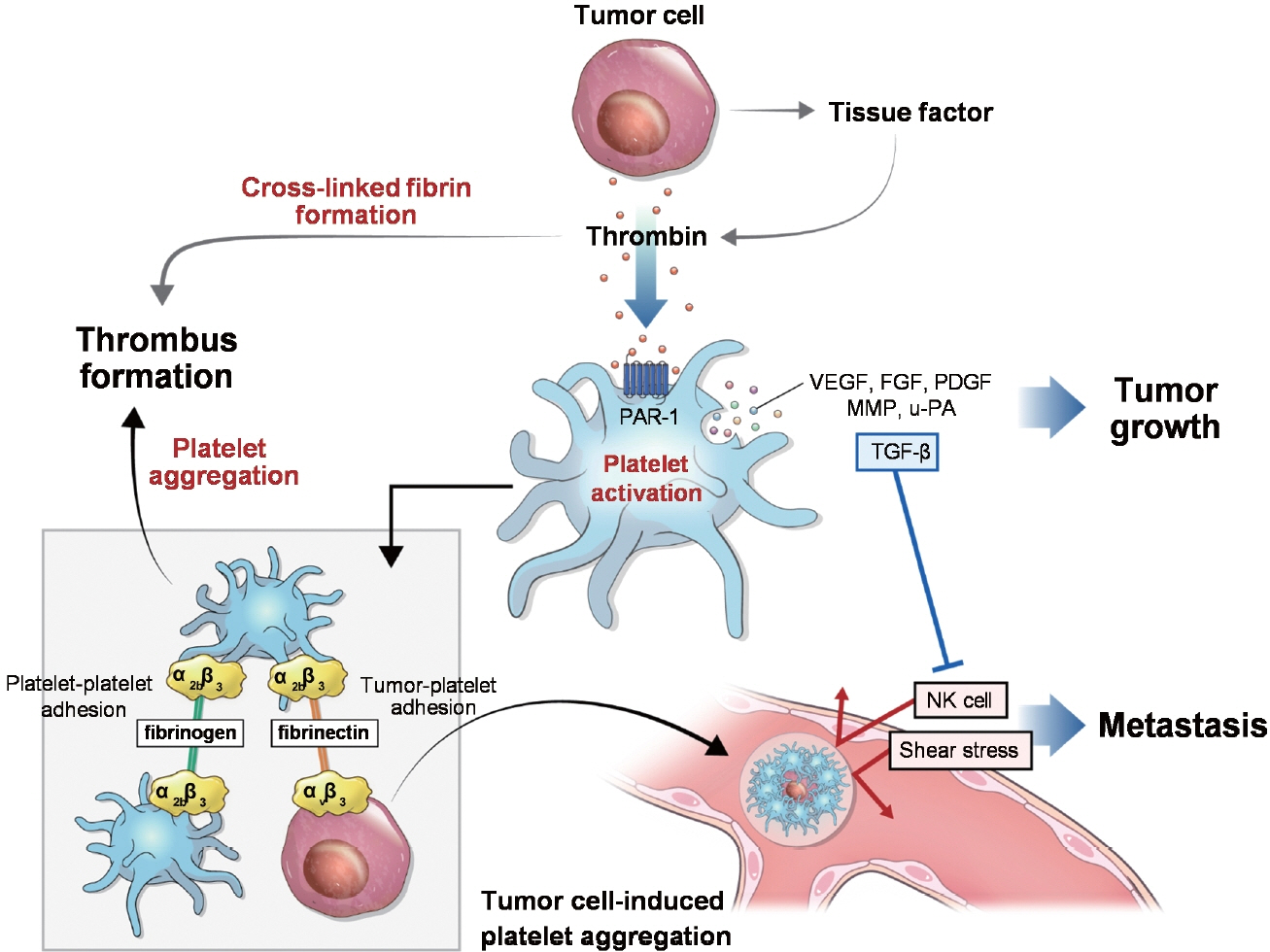
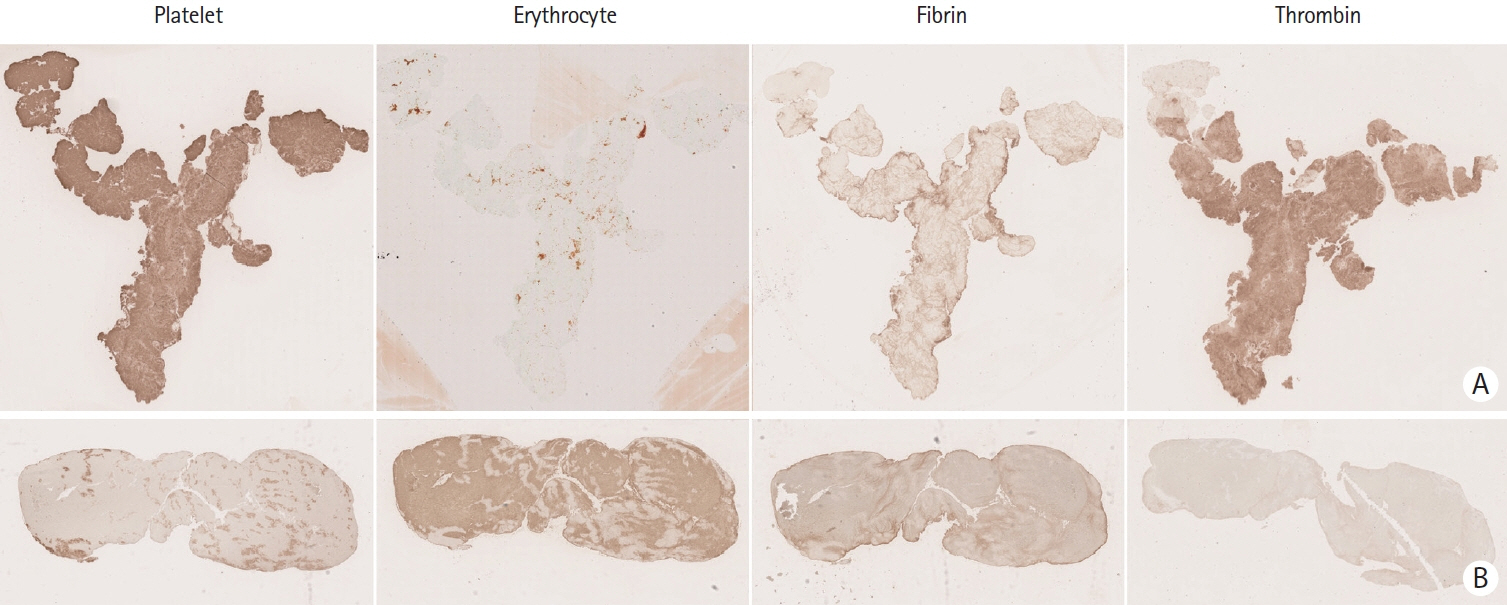
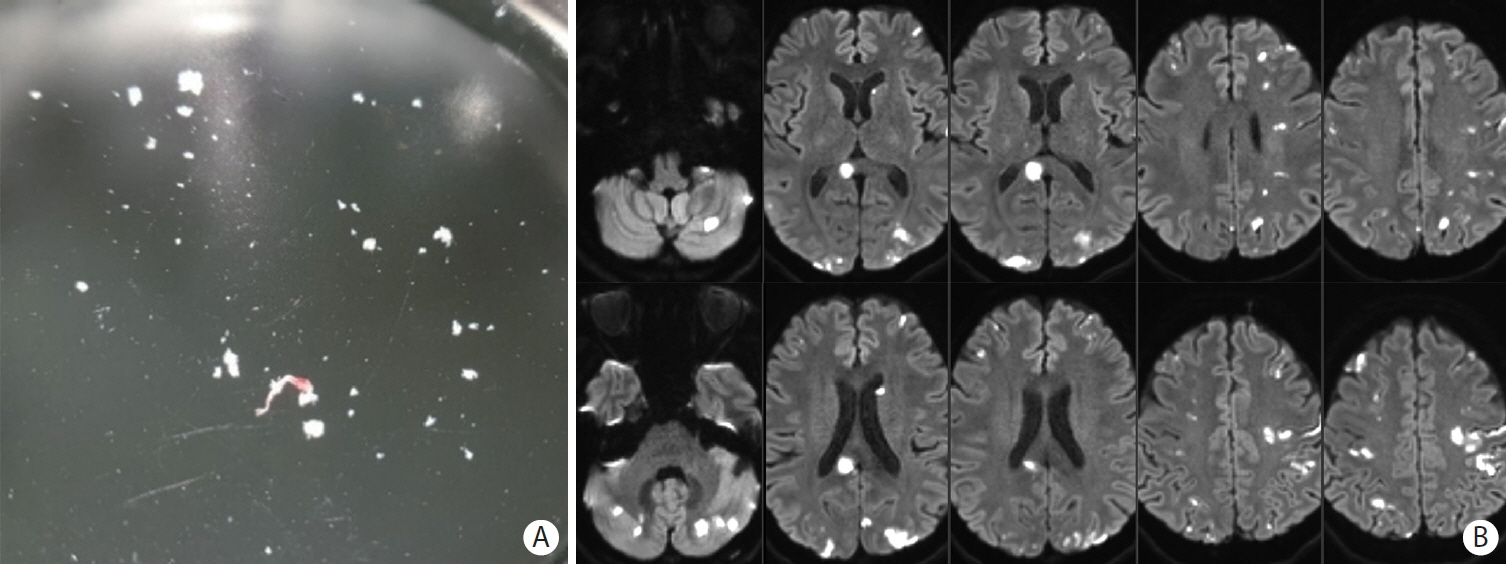
- Cancer can induce hypercoagulability, which may lead to stroke. This occurs when tumor cells activate platelets as part of their growth and metastasis. Tumor cells activate platelets by generating thrombin and expressing tissue factor, resulting in tumor cell-induced platelet aggregation. Histopathological studies of thrombi obtained during endovascular thrombectomy in patients with acute stroke and active cancer have shown a high proportion of platelets and thrombin. This underscores the crucial roles of platelets and thrombin in cancer-associated thrombosis. Cancer-associated stroke typically occurs in patients with active cancer and is characterized by distinctive features. These features include multiple infarctions across multiple vascular territories, markedly elevated blood D-dimer levels, and metastasis. The presence of cardiac vegetations on echocardiography is a robust indicator of cancer-associated stroke. Suspicion of cancer-associated stroke during endovascular thrombectomy arises when white thrombi are detected, particularly in patients with active cancer. Cancer-associated stroke is almost certain when histopathological examination of thrombi shows a very high platelet and a very low erythrocyte composition. Patients with cancer-associated stroke have high risks of mortality and recurrent stroke. However, limited data are available on the optimal treatment regimen for stroke prevention in these patients. Thrombosis mechanism in cancer is well understood, and distinct therapeutic targets involving thrombin and platelets have been identified. Therefore, direct thrombin inhibitors and/or antiplatelet agents may effectively prevent stroke recurrence. Additionally, this strategy has potential benefits in cancer treatment as accumulating evidence suggests that aspirin use reduces cancer progression, metastasis, and cancer-related mortality. However, clinical trials are necessary to assess the efficacy of this strategy involving the use of direct thrombin inhibitors and/or antiplatelet therapies.
Keyword
Figure
Reference
-
References
1. White MC, Holman DM, Boehm JE, Peipins LA, Grossman M, Henley SJ. Age and cancer risk: a potentially modifiable relationship. Am J Prev Med. 2014; 46(3 Suppl 1):S7–S15.2. Kang MJ, Jung KW, Bang SH, Choi SH, Park EH, Yun EH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2020. Cancer Res Treat. 2023; 55:385–399.
Article3. Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018; 379:2429–2437.
Article4. Sanossian N, Djabiras C, Mack WJ, Ovbiagele B. Trends in cancer diagnoses among inpatients hospitalized with stroke. J Stroke Cerebrovasc Dis. 2013; 22:1146–1150.
Article5. Jang HS, Choi J, Shin J, Chung JW, Bang OY, Kim GM, et al. The long-term effect of cancer on incident stroke: a nationwide population-based cohort study in Korea. Front Neurol. 2019; 10:52.
Article6. Navi BB, Iadecola C. Ischemic stroke in cancer patients: a review of an underappreciated pathology. Ann Neurol. 2018; 83:873–883.
Article7. Bang OY, Chung JW, Lee MJ, Seo WK, Kim GM, Ahn MJ. Cancer-related stroke: an emerging subtype of ischemic stroke with unique pathomechanisms. J Stroke. 2020; 22:1–10.
Article8. Dardiotis E, Aloizou AM, Markoula S, Siokas V, Tsarouhas K, Tzanakakis G, et al. Cancer-associated stroke: pathophysiology, detection and management (review). Int J Oncol. 2019; 54:779–796.
Article9. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/ American Stroke Association. Stroke. 2021; 52:e364–e467.10. Woock M, Martinez-Majander N, Seiffge DJ, Selvik HA, Nordanstig A, Redfors P, et al. Cancer and stroke: commonly encountered by clinicians, but little evidence to guide clinical approach. Ther Adv Neurol Disord. 2022; 15:17562864221106362.
Article11. Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011; 11:123–134.
Article12. Goubran HA, Burnouf T, Radosevic M, El-Ekiaby M. The platelet-cancer loop. Eur J Intern Med. 2013; 24:393–400.
Article13. Mege D, Aubert M, Lacroix R, Dignat-George F, Panicot-Dubois L, Dubois C. Involvement of platelets in cancers. Semin Thromb Hemost. 2019; 45:569–575.
Article14. Plantureux L, Mège D, Crescence L, Dignat-George F, Dubois C, Panicot-Dubois L. Impacts of cancer on platelet production, activation and education and mechanisms of cancer-associated thrombosis. Cancers (Basel). 2018; 10:441.
Article15. Plantureux L, Crescence L, Dignat-George F, Panicot-Dubois L, Dubois C. Effects of platelets on cancer progression. Thromb Res. 2018; 164(Suppl 1):S40–S47.
Article16. Mezouar S, Frère C, Darbousset R, Mege D, Crescence L, Dignat-George F, et al. Role of platelets in cancer and cancerassociated thrombosis: experimental and clinical evidences. Thromb Res. 2016; 139:65–76.
Article17. Connolly GC, Phipps RP, Francis CW. Platelets and cancer-associated thrombosis. Semin Oncol. 2014; 41:302–310.
Article18. Ma L, Perini R, McKnight W, Dicay M, Klein A, Hollenberg MD, et al. Proteinase-activated receptors 1 and 4 counterregulate endostatin and VEGF release from human platelets. Proc Natl Acad Sci U S A. 2005; 102:216–220.
Article19. Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell. 2018; 33:965–983.
Article20. Kwon MJ. Matrix metalloproteinases as therapeutic targets in breast cancer. Front Oncol. 2023; 12:1108695.
Article21. Strasenburg W, Józ´wicki J, Durs´lewicz J, Kuffel B, Kulczyk MP, Kowalewski A, et al. Tumor cell-induced platelet aggregation as an emerging therapeutic target for cancer therapy. Front Oncol. 2022; 12:909767.
Article22. Egan K, Cooke N, Kenny D. Living in shear: platelets protect cancer cells from shear induced damage. Clin Exp Metastasis. 2014; 31:697–704.
Article23. Leblanc R, Peyruchaud O. Metastasis: new functional implications of platelets and megakaryocytes. Blood. 2016; 128:24–31.
Article24. Weber MR, Zuka M, Lorger M, Tschan M, Torbett BE, Zijlstra A, et al. Activated tumor cell integrin αvβ3 cooperates with platelets to promote extravasation and metastasis from the blood stream. Thromb Res. 2016; 140(Suppl 1):S27–S36.
Article25. Qi C, Wei B, Zhou W, Yang Y, Li B, Guo S, et al. P-selectinmediated platelet adhesion promotes tumor growth. Oncotarget. 2015; 6:6584–6596.
Article26. Heinmöller E, Weinel RJ, Heidtmann HH, Salge U, Seitz R, Schmitz I, et al. Studies on tumor-cell-induced platelet aggregation in human lung cancer cell lines. J Cancer Res Clin Oncol. 1996; 122:735–744.
Article27. Hu L, Lee M, Campbell W, Perez-Soler R, Karpatkin S. Role of endogenous thrombin in tumor implantation, seeding, and spontaneous metastasis. Blood. 2004; 104:2746–2751.
Article28. Abdalkader M, Finitsis S, Li C, Hu W, Liu X, Ji X, et al. Endovascular versus medical management of acute basilar artery occlusion: a systematic review and meta-analysis of the randomized controlled trials. J Stroke. 2023; 25:81–91.
Article29. Morsi RZ, Elfil M, Ghaith HS, Aladawi M, Elmashad A, Kothari S, et al. Endovascular thrombectomy for large ischemic strokes: a living systematic review and meta-analysis of randomized trials. J Stroke. 2023; 25:214–222.
Article30. Heo JH, Nam HS, Kim YD, Choi JK, Kim BM, Kim DJ, et al. Pathophysiologic and therapeutic perspectives based on thrombus histology in stroke. J Stroke. 2020; 22:64–75.
Article31. Aliena-Valero A, Baixauli-Martín J, Torregrosa G, Tembl JI, Salom JB. Clot composition analysis as a diagnostic tool to gain insight into ischemic stroke etiology: a systematic review. J Stroke. 2021; 23:327–342.
Article32. Park H, Kim J, Ha J, Hwang IG, Song TJ, Yoo J, et al. Histological features of intracranial thrombi in stroke patients with cancer. Ann Neurol. 2019; 86:143–149.33. Fu CH, Chen CH, Lin YH, Lee CW, Tsai LK, Tang SC, et al. Fibrin and platelet-rich composition in retrieved thrombi hallmarks stroke with active cancer. Stroke. 2020; 51:3723–3727.
Article34. Yoo J, Kwon I, Kim S, Kim HM, Kim YD, Nam HS, et al. Coagulation factor expression and composition of arterial thrombi in cancer-associated stroke. Stroke. 2023; 54:2981–2989.
Article35. Ikeda H, Ishibashi R, Kinosada M, Uezato M, Hata H, Kaneko R, et al. Factors related to white thrombi in acute ischemic stroke in cancer patients. Neuroradiol J. 2023; 36:453–459.
Article36. Sun YE, Na HK, Kwak S, Kim YD, Nam HS, Heo JH. Different thrombus histology in a cancer patient with deep vein thrombosis and recurrent strokes. J Stroke. 2022; 24:300–302.
Article37. Heo J, Seog Y, Lee H, Lee IH, Kim S, Baek JH, et al. Automated composition analysis of thrombus from endovascular treatment in acute ischemic stroke using computer vision. J Stroke. 2022; 24:433–435.
Article38. Heo J, Lee H, Seog Y, Kim S, Baek JH, Park H, et al. Cancer prediction with machine learning of thrombi from thrombectomy in stroke: multicenter development and validation. Stroke. 2023; 54:2105–2113.
Article39. Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003; 349:146–153.
Article40. Johnson ED, Schell JC, Rodgers GM. The D-dimer assay. Am J Hematol. 2019; 94:833–839.
Article41. Weitz JI, Fredenburgh JC, Eikelboom JW. A test in context: D-dimer. J Am Coll Cardiol. 2017; 70:2411–2420.
Article42. Naito H, Nezu T, Hosomi N, Aoki S, Ueno H, Ochi K, et al. Antithrombotic therapy strategy for cancer-associated ischemic stroke: a case series of 26 patients. J Stroke Cerebrovasc Dis. 2018; 27:e206–e211.
Article43. Nam KW, Kim CK, Kim TJ, An SJ, Oh K, Ko SB, et al. Treatment of cryptogenic stroke with active cancer with a new oral anticoagulant. J Stroke Cerebrovasc Dis. 2017; 26:2976–2980.
Article44. Jang H, Lee JJ, Lee MJ, Ryoo S, Yoon CH, Kim GM, et al. Comparison of enoxaparin and warfarin for secondary prevention of cancer-associated stroke. J Oncol. 2015; 2015:502089.
Article45. Yoo J, Choi JK, Kim YD, Nam HS, Park H, Lee HS, et al. Outcome of stroke patients with cancer and nonbacterial thrombotic endocarditis. J Stroke. 2020; 22:245–253.
Article46. Asopa S, Patel A, Khan OA, Sharma R, Ohri SK. Non-bacterial thrombotic endocarditis. Eur J Cardiothorac Surg. 2007; 32:696–701.
Article47. Liu J, Frishman WH. Nonbacterial thrombotic endocarditis: pathogenesis, diagnosis, and management. Cardiol Rev. 2016; 24:244–247.48. Itzhaki Ben Zadok O, Spectre G, Leader A. Cancer-associated non-bacterial thrombotic endocarditis. Thromb Res. 2022; 213(Suppl 1):S127–S132.
Article49. Deppisch LM, Fayemi AO. Non-bacterial thrombotic endocarditis: clinicopathologic correlations. Am Heart J. 1976; 92:723–729.50. Navi BB, Singer S, Merkler AE, Cheng NT, Stone JB, Kamel H, et al. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology. 2014; 83:26–33.
Article51. Lee MJ, Chung JW, Ahn MJ, Kim S, Seok JM, Jang HM, et al. Hypercoagulability and mortality of patients with stroke and active cancer: the OASIS-cancer study. J Stroke. 2017; 19:77–87.
Article52. Nam KW, Kim CK, Kim TJ, An SJ, Oh K, Mo H, et al. Predictors of 30-day mortality and the risk of recurrent systemic thromboembolism in cancer patients suffering acute ischemic stroke. PLoS One. 2017; 12:e0172793.53. Yoo J, Nam HS, Kim YD, Lee HS, Heo JH. Short-term outcome of ischemic stroke patients with systemic malignancy. Stroke. 2019; 50:507–511.
Article54. Fujinami J, Ohara T, Kitani-Morii F, Tomii Y, Makita N, Yamada T, et al. Cancer-associated hypercoagulation increases the risk of early recurrent stroke in patients with active cancer. Cerebrovasc Dis. 2018; 46:46–51.
Article55. Nakajima S, Kawano H, Yamashiro K, Tanaka R, Kameda T, Kurita N, et al. Post-treatment plasma D-dimer levels are associated with short-term outcomes in patients with cancerassociated stroke. Front Neurol. 2022; 13:868137.
Article56. Verschoof MA, Groot AE, de Bruijn SFTM, Roozenbeek B, van der Worp HB, Dippel DWJ, et al. Clinical outcome after endovascular treatment in patients with active cancer and ischemic stroke: a MR CLEAN registry substudy. Neurology. 2022; 98:e993–e1001.57. Aboul-Nour H, Maraey A, Jumah A, Khalil M, Elzanaty AM, Elsharnoby H, et al. Mechanical thrombectomy for acute ischemic stroke in metastatic cancer patients: a nationwide crosssectional analysis. J Stroke. 2023; 25:119–125.
Article58. Yoo J, Kim YD, Park H, Kim BM, Bang OY, Kim HC, et al. Immediate and long-term outcomes of reperfusion therapy in patients with cancer. Stroke. 2021; 52:2026–2034.
Article59. Navi BB, Zhang C, Sherman CP, Genova R, LeMoss NM, Kamel H, et al. Ischemic stroke with cancer: hematologic and embolic biomarkers and clinical outcomes. J Thromb Haemost. 2022; 20:2046–2057.
Article60. Thun MJ, Namboodiri MM, Heath CW Jr. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991; 325:1593–1596.
Article61. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med. 1994; 121:241–246.
Article62. Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, et al. Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995; 333:609–614.
Article63. Smalley W, Ray WA, Daugherty J, Griffin MR. Use of nonsteroidal anti-inflammatory drugs and incidence of colorectal cancer: a population-based study. Arch Intern Med. 1999; 159:161–166.
Article64. Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003; 348:891–899.
Article65. Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003; 348:883–890.
Article66. Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007; 369:1603–1613.
Article67. Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012; 13:518–527.
Article68. Simon TG, Duberg AS, Aleman S, Chung RT, Chan AT, Ludvigsson JF. Association of aspirin with hepatocellular carcinoma and liver-related mortality. N Engl J Med. 2020; 382:1018–1028.
Article69. Elwood PC, Morgan G, Delon C, Protty M, Galante J, Pickering J, et al. Aspirin and cancer survival: a systematic review and meta-analyses of 118 observational studies of aspirin and 18 cancers. Ecancermedicalscience. 2021; 15:1258.
Article70. Ma S, Qu G, Sun C, Liu H, Jiang Y, Li N, et al. Does aspirin reduce the incidence, recurrence, and mortality of hepatocellular carcinoma? A GRADE-assessed systematic review and dose-response meta-analysis. Eur J Clin Pharmacol. 2023; 79:39–61.
Article71. Wang L, Zhang R, Yu L, Xiao J, Zhou X, Li X, et al. Aspirin use and common cancer risk: a meta-analysis of cohort studies and randomized controlled trials. Front Oncol. 2021; 11:690219.
Article72. Zeng RW, Yong JN, Tan DJH, Fu CE, Lim WH, Xiao J, et al. Meta-analysis: chemoprevention of hepatocellular carcinoma with statins, aspirin and metformin. Aliment Pharmacol Ther. 2023; 57:600–609.73. Mädge JC, Stallmach A, Kleebusch L, Schlattmann P. Metaanalysis of aspirin-guided therapy of colorectal cancer. J Cancer Res Clin Oncol. 2022; 148:1407–1417.
Article74. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007; 357:2001–2015.
Article75. Hicks BM, Murray LJ, Hughes C, Cardwell CR. Clopidogrel use and cancer-specific mortality: a population-based cohort study of colorectal, breast and prostate cancer patients. Pharmacoepidemiol Drug Saf. 2015; 24:830–840.
Article76. Kuan YC, Huang KW, Lin CL, Luo JC, Kao CH. Effects of aspirin or clopidogrel on colorectal cancer chemoprevention in patients with type 2 diabetes mellitus. Cancers (Basel). 2019; 11:1468.
Article77. Rodríguez-Miguel A, García-Rodríguez LA, Gil M, Montoya H, Rodríguez-Martín S, de Abajo FJ. Clopidogrel and low-dose aspirin, alone or together, reduce risk of colorectal cancer. Clin Gastroenterol Hepatol. 2019; 17:2024–2033.e2.
Article78. Cho MS, Noh K, Haemmerle M, Li D, Park H, Hu Q, et al. Role of ADP receptors on platelets in the growth of ovarian cancer. Blood. 2017; 130:1235–1242.
Article79. Gebremeskel S, LeVatte T, Liwski RS, Johnston B, Bezuhly M. The reversible P2Y12 inhibitor ticagrelor inhibits metastasis and improves survival in mouse models of cancer. Int J Cancer. 2015; 136:234–240.
Article80. Mezouar S, Darbousset R, Dignat-George F, Panicot-Dubois L, Dubois C. Inhibition of platelet activation prevents the P-selectin and integrin-dependent accumulation of cancer cell microparticles and reduces tumor growth and metastasis in vivo. Int J Cancer. 2015; 136:462–475.
Article81. Hua H, Zhang H, Kong Q, Wang J, Jiang Y. Complex roles of the old drug aspirin in cancer chemoprevention and therapy. Med Res Rev. 2019; 39:114–145.
Article82. Mitrugno A, Sylman JL, Rigg RA, Tassi Yunga S, Shatzel JJ, Williams CD, et al. Carpe low-dose aspirin: the new anti-cancer face of an old anti-platelet drug. Platelets. 2018; 29:773–778.
Article83. Kawano H, Honda Y, Amano T, Okano H, Suzuki R, Torii M, et al. Subcutaneous heparin therapy for patients with cancerassociated stroke. J Stroke Cerebrovasc Dis. 2019; 28:399–404.
Article84. Yamaura G, Ito T, Miyaji Y, Ueda N, Nakae Y, Momoo T, et al. Therapeutic efficacy of heparin and direct factor Xa inhibitors in cancer-associated cryptogenic ischemic stroke with venous thromboembolism. Thromb Res. 2021; 206:99–103.
Article85. Navi BB, Marshall RS, Bobrow D, Singer S, Stone JB, DeSancho MT, et al. Enoxaparin vs aspirin in patients with cancer and ischemic stroke: the teach pilot randomized clinical trial. JAMA Neurol. 2018; 75:379–381.
Article86. Chung JW, Hwang J, Kim HJ, Seo WK, Ahn MJ, Saver JL, et al. Edoxaban for the treatment of hypercoagulability and cerebral thromboembolism associated with cancer: a randomized clinical trial of biomarker targets. Int J Stroke. 2024 Mar 21 [Epub]. Available from: https://doi.org/10.1177/17474930241239266.
Article87. Martinez-Majander N, Ntaios G, Liu YY, Ylikotila P, Joensuu H, Saarinen J, et al. Rivaroxaban versus aspirin for secondary prevention of ischaemic stroke in patients with cancer: a subgroup analysis of the NAVIGATE ESUS randomized trial. Eur J Neurol. 2020; 27:841–848.88. Conen D, Wong JA, Sandhu RK, Cook NR, Lee IM, Buring JE, et al. Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol. 2016; 14:389–396.
Article89. Menichelli D, Vicario T, Ameri P, Toma M, Violi F, Pignatelli P, et al. Cancer and atrial fibrillation: epidemiology, mechanisms, and anticoagulation treatment. Prog Cardiovasc Dis. 2021; 66:28–36.
Article90. Hung YP, Hu YW, Liu CJ, Lin YJ, Chang SL, Lo LW, et al. Risk and predictors of subsequent cancers of patients with newlydiagnosed atrial fibrillation - a nationwide population-based study. Int J Cardiol. 2019; 296:81–86.
Article91. Atterman A, Asplund K, Friberg L, Engdahl J. Use of oral anticoagulants after ischaemic stroke in patients with atrial fibrillation and cancer. J Intern Med. 2020; 288:457–468.
Article92. Kim K, Lee YJ, Kim TH, Uhm JS, Pak HN, Lee MH, et al. Effect of non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with newly diagnosed cancer. Korean Circ J. 2018; 48:406–417.
Article93. Shah S, Norby FL, Datta YH, Lutsey PL, MacLehose RF, Chen LY, et al. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv. 2018; 2:200–209.
Article94. Yang P, Zhu D, Xu X, Shen W, Wang C, Jiang Y, et al. Efficacy and safety of oral anticoagulants in atrial fibrillation patients with cancer—a network meta-analysis. Heart Fail Rev. 2020; 25:823–831.
Article95. Chen Y, Mao M, Chang J, Yan J, Yang T, Liu Y, et al. Safety and efficacy of new oral anticoagulants compared to those of warfarin in AF patients with cancer: a meta-analysis of randomized clinical trials and observational studies. Eur J Clin Pharmacol. 2021; 77:849–857.
Article96. Chan YH, Chao TF, Lee HF, Chen SW, Li PR, Liu JR, et al. Clinical outcomes in atrial fibrillation patients with a history of cancer treated with non-vitamin K antagonist oral anticoagulants: a nationwide cohort study. Stroke. 2021; 52:3132–3141.
Article97. Deitelzweig S, Keshishian AV, Zhang Y, Kang A, Dhamane AD, Luo X, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients with active cancer. JACC CardioOncol. 2021; 3:411–424.98. Garg A, Chopra S, Starr M, Rocha M, Dawod J, Leira E, et al. In-hospital outcomes and recurrence of acute ischemic stroke in patients with solid organ malignancy. Neurology. 2022; 99:e393–e401.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Outcome Measure and Efficacy Analysis in Stroke Clinical Trials
- Dural sinus thrombosis identified by point-of-care ultrasound
- Cancer-Related Stroke: An Emerging Subtype of Ischemic Stroke with Unique Pathomechanisms
- Antithrombotic and Neuroprotective Therapy in Acute Ischemic Stroke
- Sleep Disorders after Stroke