Clin Exp Otorhinolaryngol.
2024 May;17(2):122-136. 10.21053/ceo.2023.00025.
MicroRNA-145-5p Regulates the Epithelial-Mesenchymal Transition in Nasal Polyps by Targeting Smad3
- Affiliations
-
- 1Department of Otorhinolaryngology, The First Affiliated Hospital of Soochow University, Suzhou, China
- 2Department of Orthopedics, The First Affiliated Hospital of Soochow University, Suzhou, China
- 3Department of Oncology, Affiliated Haian Hospital of Nantong University, Nantong, China
- KMID: 2556003
- DOI: http://doi.org/10.21053/ceo.2023.00025
Abstract
Objectives
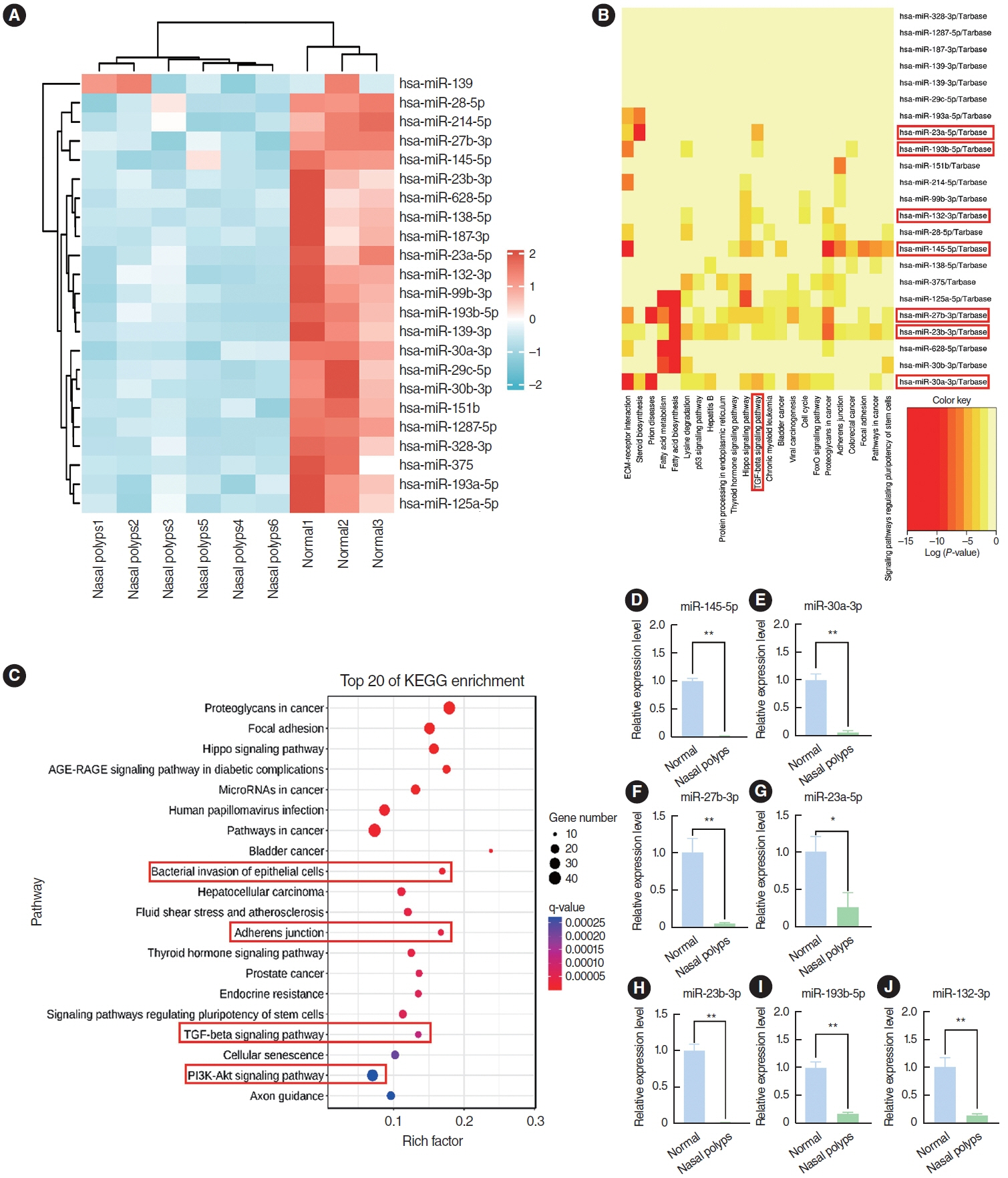
. The annual prevalence of chronic rhinosinusitis (CRS) is increasing, and the lack of effective treatments imposes a substantial burden on both patients and society. The formation of nasal polyps in patients with CRS is closely related to tissue remodeling, which is largely driven by the epithelial-mesenchymal transition (EMT). MicroRNA (miRNA) plays a pivotal role in the pathogenesis of numerous diseases through the miRNA-mRNA regulatory network; however, the specific mechanism of the miRNAs involved in the formation of nasal polyps remains unclear.
Methods
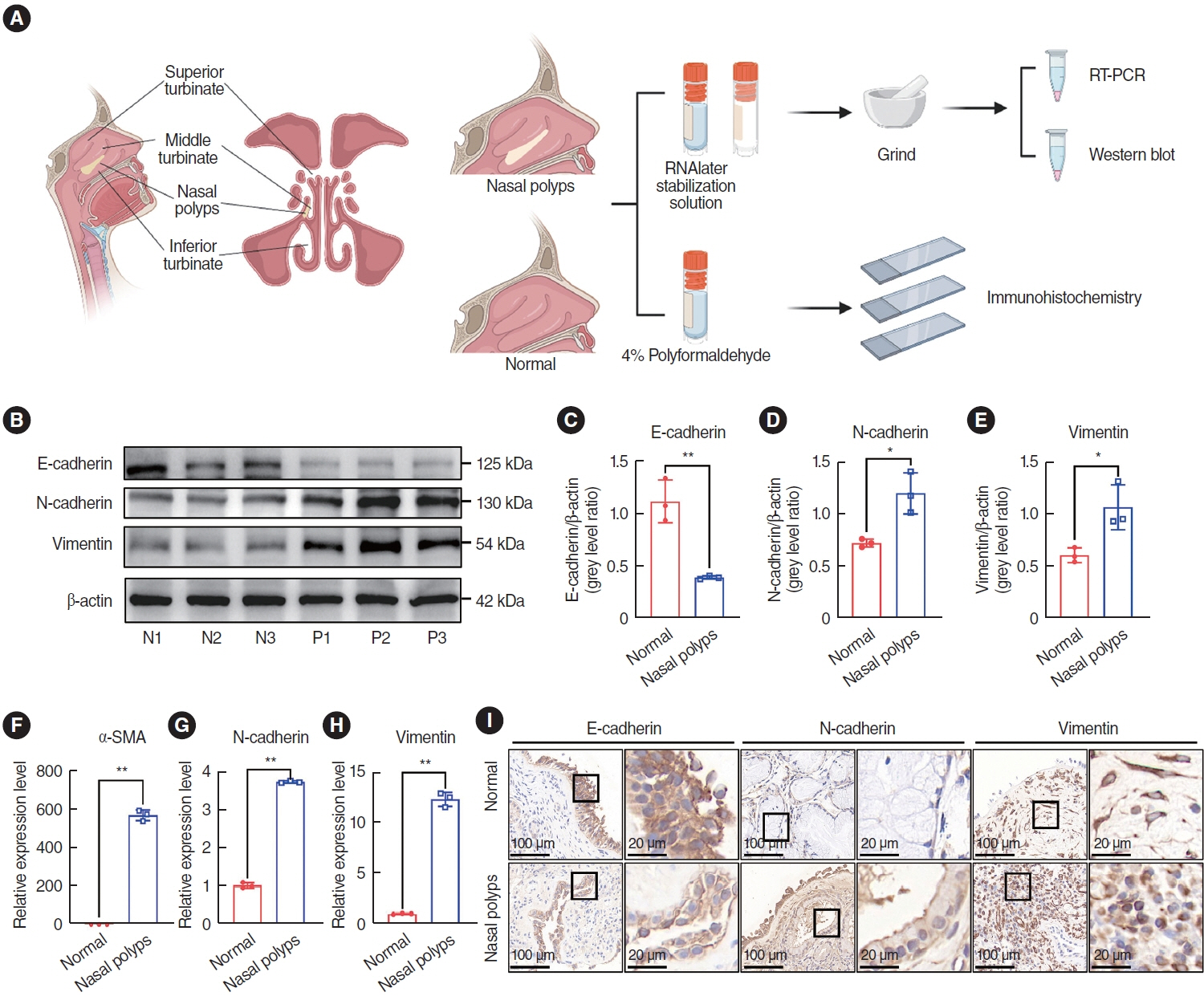
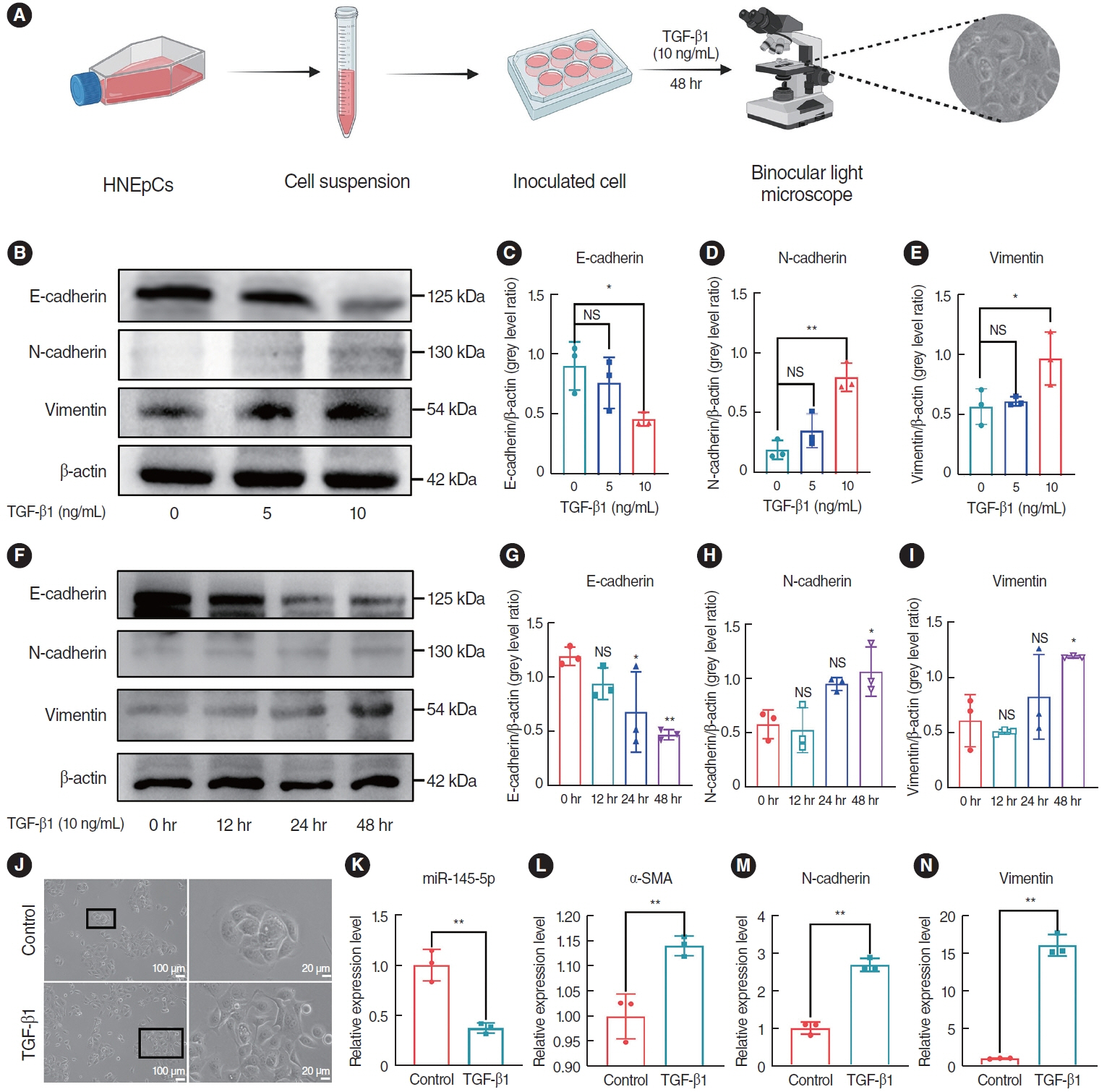
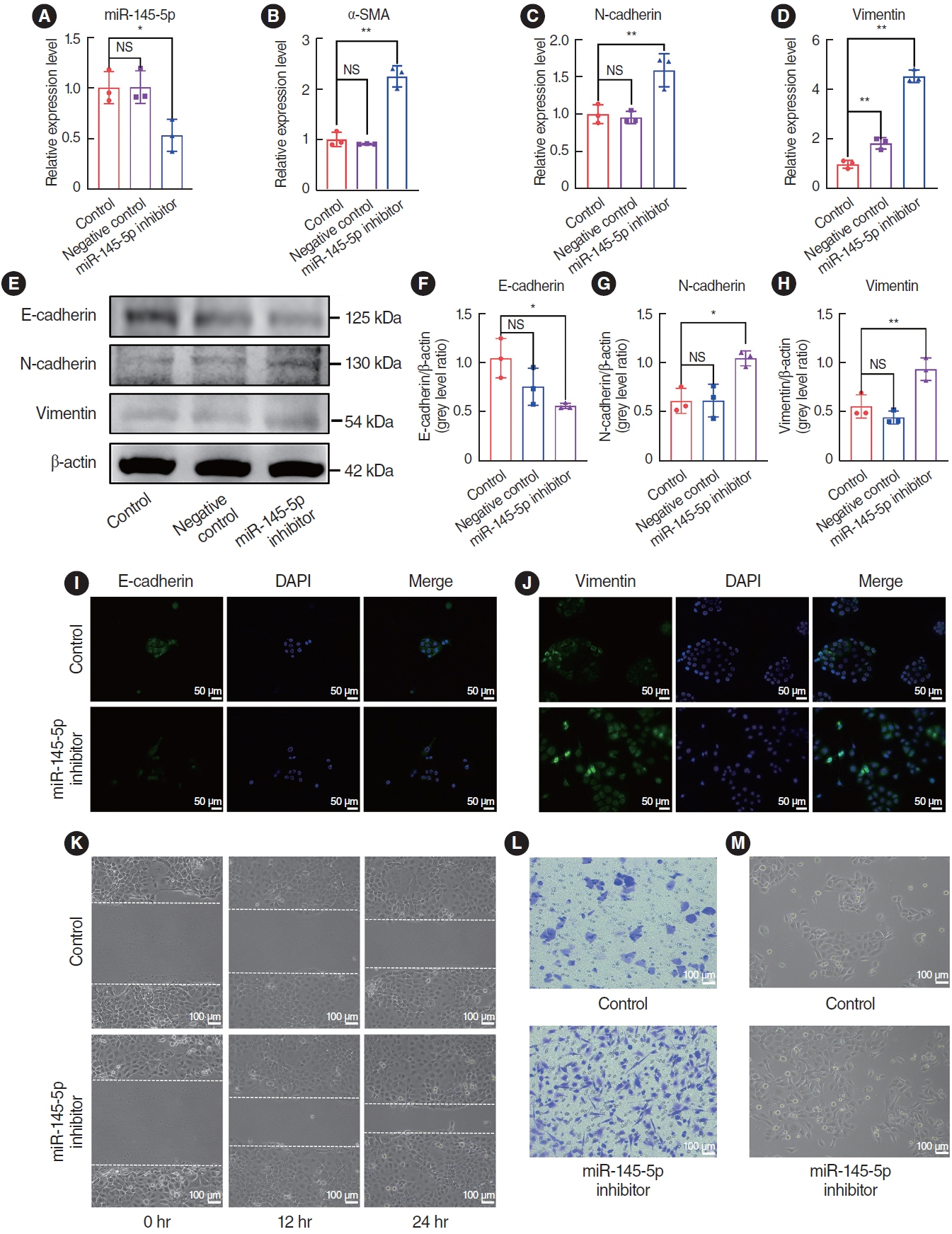
. The expression of EMT markers and Smad3 were detected using western blots, quantitative real-time polymerase chain reaction, and immunohistochemical and immunofluorescence staining. Differentially expressed genes in nasal polyps and normal tissues were screened through the Gene Expression Omnibus database. To predict the target genes of miR-145-5p, three different miRNA target prediction databases were used. The migratory ability of cells was evaluated using cell migration assay and wound healing assays.
Results
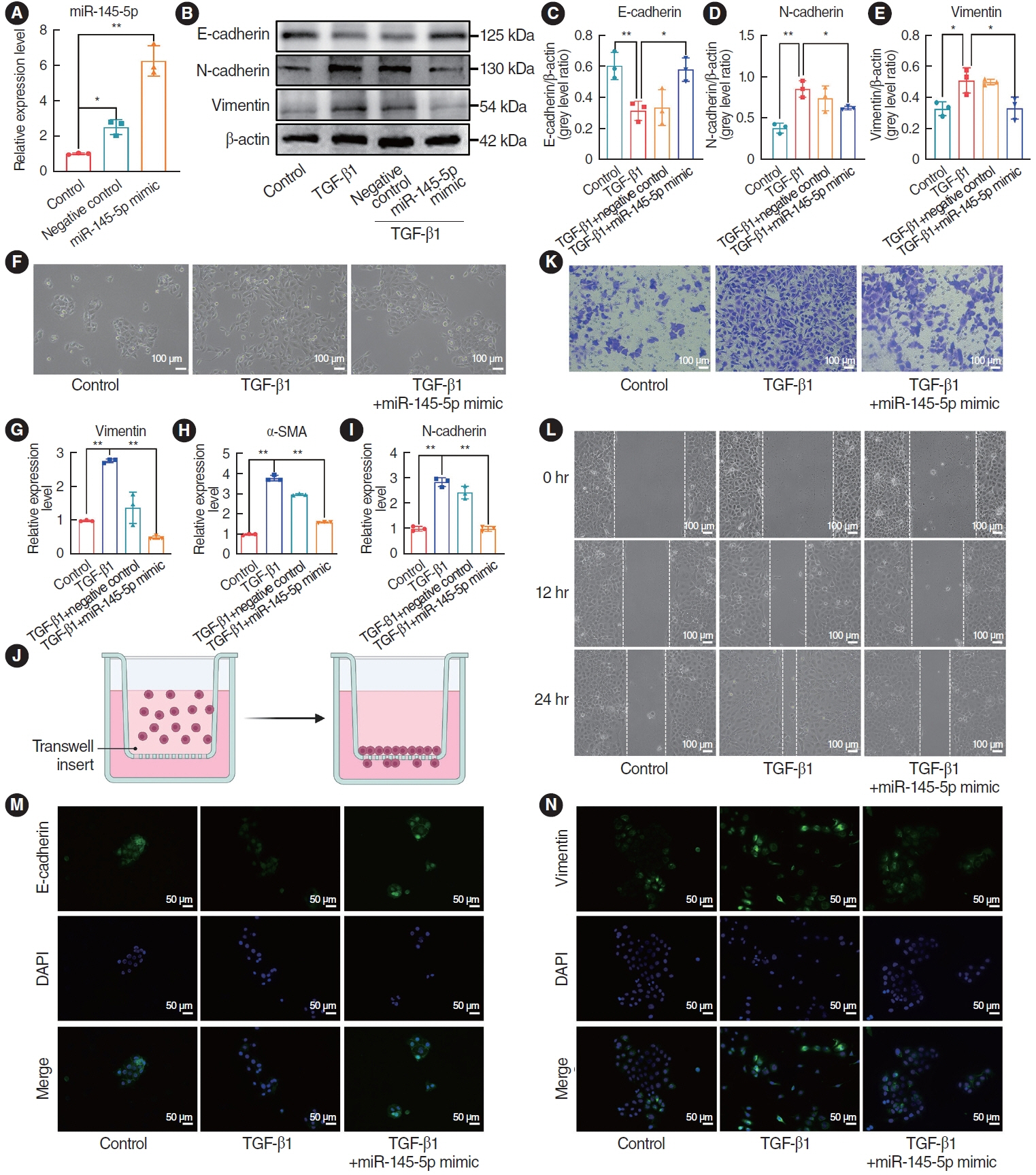
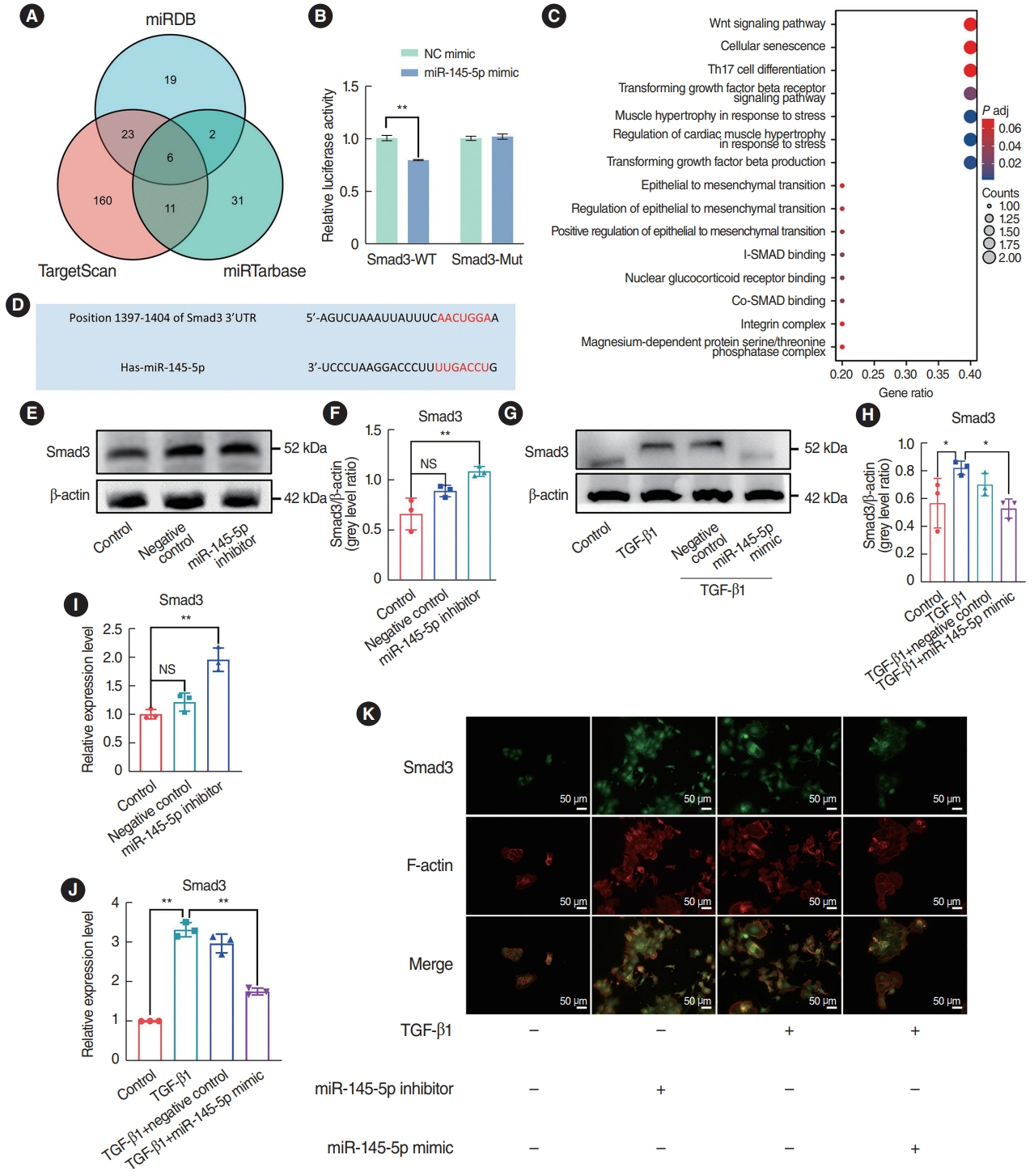
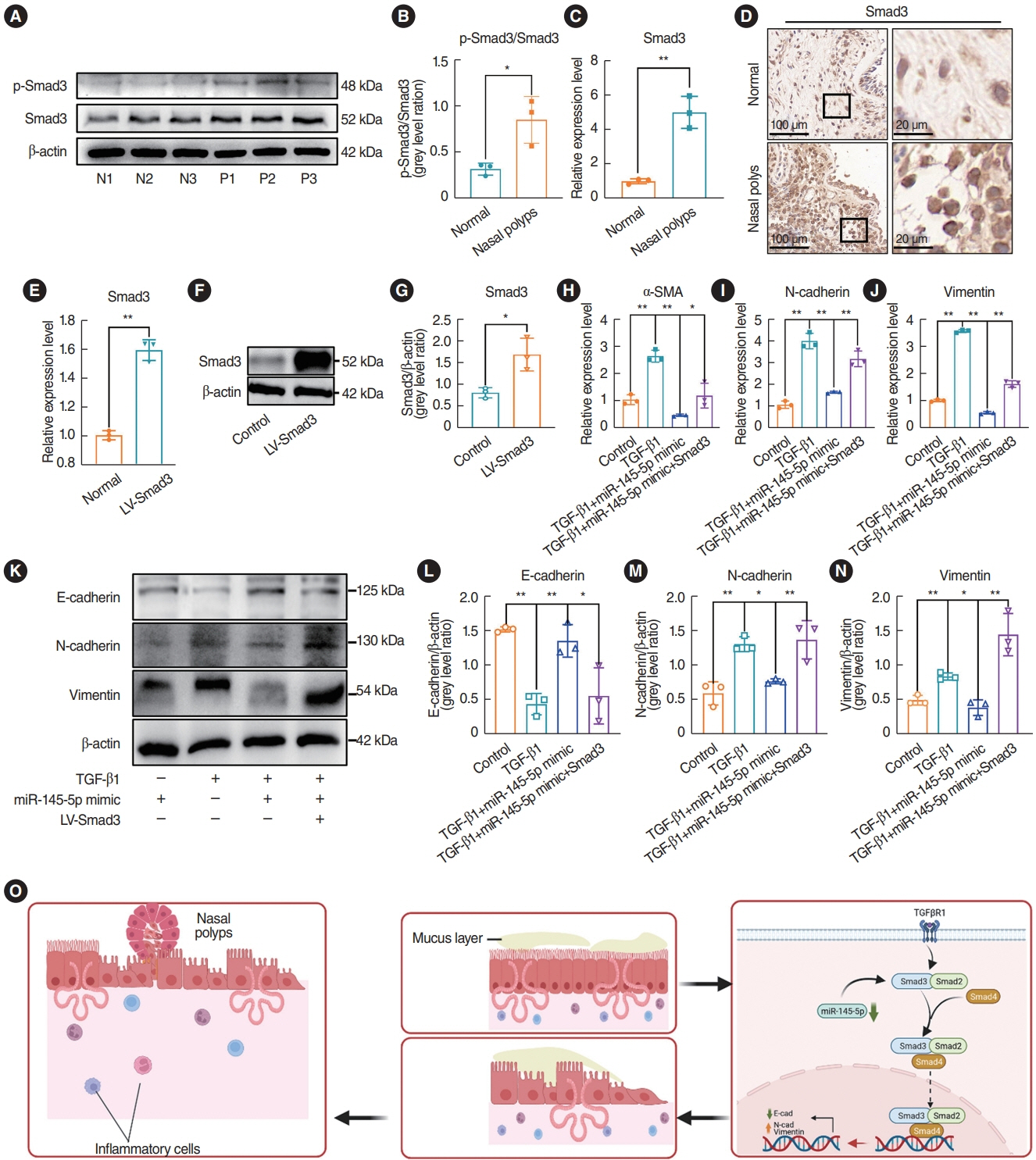
. miR-145-5p was associated with the EMT process and was significantly downregulated in nasal polyp tissues. In vitro experiments revealed that the downregulation of miR-145-5p promoted EMT. Conversely, increasing miR-145-5p levels reversed the EMT induced by transforming growth factor-β1. Bioinformatics analysis suggested that miR-145-5p targets Smad3. Subsequent experiments confirmed that miR-145-5p inhibits Smad3 expression.
Conclusion
. Overall, miR-145-5p is a promising target to inhibit nasal polyp formation, and the findings of this study provide a theoretical basis for nanoparticle-mediated miR-145-5p delivery for the treatment of nasal polyps.
Figure
Cited by 1 articles
-
Reprogramming Macrophage Phenotypes With Photobiomodulation for Improved Inflammation Control in ENT Organ Tissues
Ken Woo, Yeon Soo Kim, Celine Abueva, Seung Hoon Woo
Clin Exp Otorhinolaryngol. 2025;18(1):1-13. doi: 10.21053/ceo.2024.00286.
Reference
-
1. Bachert C, Marple B, Schlosser RJ, Hopkins C, Schleimer RP, Lambrecht BN, et al. Adult chronic rhinosinusitis. Nat Rev Dis Primers. 2020; Oct. 6(1):86.
Article2. Fujieda S, Imoto Y, Kato Y, Ninomiya T, Tokunaga T, Tsutsumiuchi T, et al. Eosinophilic chronic rhinosinusitis. Allergol Int. 2019; Oct. 68(4):403–12.
Article3. Cardell LO, Stjarne P, Jonstam K, Bachert C. Endotypes of chronic rhinosinusitis: impact on management. J Allergy Clin Immunol. 2020; Mar. 145(3):752–6.
Article4. Hopkins C. Chronic rhinosinusitis with nasal polyps. N Engl J Med. 2019; Jul. 381(1):55–63.
Article5. Sedaghat AR, Kuan EC, Scadding GK. Epidemiology of chronic rhinosinusitis: prevalence and risk factors. J Allergy Clin Immunol Pract. 2022; Jun. 10(6):1395–403.
Article6. Wahid NW, Smith R, Clark A, Salam M, Philpott CM. The socioeconomic cost of chronic rhinosinusitis study. Rhinology. 2020; Apr. 58(2):112–25.
Article7. Bracken CP, Goodall GJ. The many regulators of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2022; Feb. 23(2):89–90.
Article8. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019; Feb. 20(2):69–84.
Article9. Manfioletti G, Fedele M. Epithelial-mesenchymal transition (EMT) 2021. Int J Mol Sci. 2022; May. 23(10):5848.
Article10. Feng YL, Chen DQ, Vaziri ND, Guo Y, Zhao YY. Small molecule inhibitors of epithelial-mesenchymal transition for the treatment of cancer and fibrosis. Med Res Rev. 2020; Jan. 40(1):54–78.
Article11. Lee H, Hwang-Bo H, Ji SY, Kim MY, Kim SY, Park C, et al. Diesel particulate matter2.5 promotes epithelial-mesenchymal transition of human retinal pigment epithelial cells via generation of reactive oxygen species. Environ Pollut. 2020; Jul. 262:114301.
Article12. Yuan FL, Sun ZL, Feng Y, Liu SY, Du Y, Yu S, et al. Epithelial-mesenchymal transition in the formation of hypertrophic scars and keloids. J Cell Physiol. 2019; Dec. 234(12):21662–9.
Article13. Lee HW, Jose CC, Cuddapah S. Epithelial-mesenchymal transition: insights into nickel-induced lung diseases. Semin Cancer Biol. 2021; Nov. 76:99–109.
Article14. Lee M, Lim S, Kim YS, Khalmuratova R, Shin SH, Kim I, et al. DEPinduced ZEB2 promotes nasal polyp formation via epithelial-tomesenchymal transition. J Allergy Clin Immunol. 2022; Jan. 149(1):340–57.
Article15. Jiang W, Zhou C, Ma C, Cao Y, Hu G, Li H. TGF-β1 induces epithelial-to-mesenchymal transition in chronic rhinosinusitis with nasal polyps through microRNA-182. Asian Pac J Allergy Immunol. 2024; Mar. 42(1):61–73.
Article16. Chiarella E, Lombardo N, Lobello N, Aloisio A, Aragona T, Pelaia C, et al. Nasal polyposis: insights in epithelial-mesenchymal transition and differentiation of polyp mesenchymal stem cells. Int J Mol Sci. 2020; Sep. 21(18):6878.
Article17. Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018; Apr. 141(4):1202–7.
Article18. Kadkhoda S, Ghafouri-Fard S. Function of miRNA-145-5p in the pathogenesis of human disorders. Pathol Res Pract. 2022; Mar. 231:153780.
Article19. Suzuki HI. MicroRNA control of TGF-β signaling. Int J Mol Sci. 2018; Jun. 19(7):1901.
Article20. Zubrzycka A, Migdalska-Sek M, Jedrzejczyk S, Brzezianska-Lasota E. The expression of TGF-β1, SMAD3, ILK and miRNA-21 in the ectopic and eutopic endometrium of women with endometriosis. Int J Mol Sci. 2023; Jan. 24(3):2453.
Article21. Pan G, Liu Y, Shang L, Zhou F, Yang S. EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun (Lond). 2021; Mar. 41(3):199–217.
Article22. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017; Mar. 16(3):203–22.
Article23. He B, Zhao Z, Cai Q, Zhang Y, Zhang P, Shi S, et al. miRNA-based biomarkers, therapies, and resistance in cancer. Int J Biol Sci. 2020; 16(14):2628–47.
Article24. Lee SW, Paoletti C, Campisi M, Osaki T, Adriani G, Kamm RD, et al. MicroRNA delivery through nanoparticles. J Control Release. 2019; Nov. 313:80–95.
Article25. Wang Y, Cao Y. miR-145-5p inhibits psoriasis progression by regulating the Wnt/β-catenin pathway. Am J Transl Res. 2021; 13(9):10439–48.26. Shen W, Wang Y, Wang D, Zhou H, Zhang H, Li L. miR-145-5p attenuates hypertrophic scar via reducing Smad2/Smad3 expression. Biochem Biophys Res Commun. 2020; Jan. 521(4):1042–8.
Article27. Yu J, Kang X, Xiong Y, Luo Q, Dai D, Ye J. Gene expression profiles of circular RNAs and MicroRNAs in chronic rhinosinusitis with nasal polyps. Front Mol Biosci. 2021; 8:643504.
Article28. Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020; Feb. 58(Suppl S29):1–464.29. Qin D, Liu P, Zhou H, Jin J, Gong W, Liu K, et al. TIM-4 in macrophages contributes to nasal polyp formation through the TGF-β1- mediated epithelial to mesenchymal transition in nasal epithelial cells. Front Immunol. 2022; 13:941608.30. Kato A, Schleimer RP, Bleier BS. Mechanisms and pathogenesis of chronic rhinosinusitis. J Allergy Clin Immunol. 2022; May. 149(5):1491–503.
Article31. Zhong B, Seah JJ, Liu F, Ba L, Du J, Wang Y. The role of hypoxia in the pathophysiology of chronic rhinosinusitis. Allergy. 2022; Nov. 77(11):3217–32.
Article32. Lin YT, Wu KJ. Epigenetic regulation of epithelial-mesenchymal transition: focusing on hypoxia and TGF-β signaling. J Biomed Sci. 2020; Mar. 27(1):39.
Article33. Kilikevicius A, Meister G, Corey DR. Reexamining assumptions about miRNA-guided gene silencing. Nucleic Acids Res. 2022; Jan. 50(2):617–34.
Article34. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelialmesenchymal transition. Nat Rev Mol Cell Biol. 2014; Mar. 15(3):178–96.
Article35. Gu W, Yuan Y, Wang L, Yang H, Li S, Tang Z, et al. Long non-coding RNA TUG1 promotes airway remodelling by suppressing the miR145-5p/DUSP6 axis in cigarette smoke-induced COPD. J Cell Mol Med. 2019; Nov. 23(11):7200–9.
Article36. Qiu ZK, Yang E, Yu NZ, Zhang MZ, Zhang WC, Si LB, et al. The biomarkers associated with epithelial-mesenchymal transition in human keloids. Burns. 2024; Mar. 50(2):474–87.
Article37. Knight DA, Grainge CL, Stick SM, Kicic A, Schuliga M. Epithelial mesenchymal transition in respiratory disease: fact or fiction. Chest. 2020; Jun. 157(6):1591–6.
Article38. Wang Z, Lin D, Zhao Y, Liu H, Yang T, Li A. MiR-214 expression is elevated in chronic rhinosinusitis mucosa and regulates lipopolysaccharide-mediated responses in undifferentiated human nasal epithelial cell culture. Am J Rhinol Allergy. 2023; Jul. 37(4):391–401.
Article39. Li X, Li C, Zhu G, Yuan W, Xiao ZA. TGF-β1 induces epithelial-mesenchymal transition of chronic sinusitis with nasal polyps through MicroRNA-21. Int Arch Allergy Immunol. 2019; 179(4):304–19.
Article40. Cheng J, Chen J, Zhao Y, Yang J, Xue K, Wang Z. MicroRNA-761 suppresses remodeling of nasal mucosa and epithelial-mesenchymal transition in mice with chronic rhinosinusitis through LCN2. Stem Cell Res Ther. 2020; Apr. 11(1):151.
Article41. Mei LL, Wang WJ, Qiu YT, Xie XF, Bai J, Shi ZZ. miR-145-5p suppresses tumor cell migration, invasion and epithelial to mesenchymal transition by regulating the Sp1/NF-κB signaling pathway in esophageal squamous cell carcinoma. Int J Mol Sci. 2017; Aug. 18(9):1833.
Article42. Cheng X, Shen T, Liu P, Fang S, Yang Z, Li Y, et al. mir-145-5p is a suppressor of colorectal cancer at early stage, while promotes colorectal cancer metastasis at late stage through regulating AKT signaling evoked EMT-mediated anoikis. BMC Cancer. 2022; Nov. 22(1):1151.
Article43. Zhong B, Sun S, Tan KS, Ong HH, Du J, Liu F, et al. Hypoxia-inducible factor 1α activates the NLRP3 inflammasome to regulate epithelial differentiation in chronic rhinosinusitis. J Allergy Clin Immunol. 2023; Dec. 152(6):1444–59.
Article44. Jo S, Jin BJ, Lee SH, Jo HR, Park JM, Hwang KG, et al. Eosinophil-derived interferon-γ drives transmembrane protein 119-induced new bone formation in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2023; Mar. 13(3):242–54.
Article45. van der Lans RJ, Otten JJ, Adriaensen GF, Hoven DR, Benoist LB, Fokkens WJ, et al. Two-year results of tapered dupilumab for CRSwNP demonstrates enduring efficacy established in the first 6months. Allergy. 2023; Oct. 78(10):2684–97.
Article46. Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013; Nov. 12(11):967–77.
Article47. Kara G, Calin GA, Ozpolat B. RNAi-based therapeutics and tumor targeted delivery in cancer. Adv Drug Deliv Rev. 2022; Mar. 182:114113.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to “MicroRNA-370 Regulates Cellepithelial-Mesenchymal Transition, Migration, Invasion, and Prognosis of Hepatocellular Carcinoma by Targeting GUCD1†by He Y and He X (Yonsei Med J 2019;60:267–276.)
- Targeting epithelial-mesenchymal transition pathway in hepatocellular carcinoma
- MiR-182-5p Mediated by Exosomes Derived From Bone Marrow Mesenchymal Stem Cell Attenuates Inflammatory Responses by Targeting TLR4 in a Mouse Model of Myocardial Infraction
- Kartogenin Promotes the BMSCs Chondrogenic Differentiation in Osteoarthritis by Down-Regulation of miR-145-5p Targeting Smad4 Pathway
- Downregulation of TET2 Contributes to Nasal Polypogenesis Through Hypoxia-Inducible Factor 1α-Mediated Epithelial-to-Mesenchymal Transition