Clin Exp Otorhinolaryngol.
2024 Feb;17(1):64-77. 10.21053/ceo.2023.01340.
Downregulation of TET2 Contributes to Nasal Polypogenesis Through Hypoxia-Inducible Factor 1α-Mediated Epithelial-to-Mesenchymal Transition
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Renmin Hospital of Wuhan University, Wuhan, China
- 2Department of Rhinology and Allergy, Renmin Hospital of Wuhan University, Wuhan, China
- 3Hubei Province Key Laboratory of Allergy and Immunology, Wuhan, China
- 4Research Institute of Otolaryngology-Head and Neck Surgery, Renmin Hospital of Wuhan University, Wuhan, China
- KMID: 2553057
- DOI: http://doi.org/10.21053/ceo.2023.01340
Abstract
Objectives
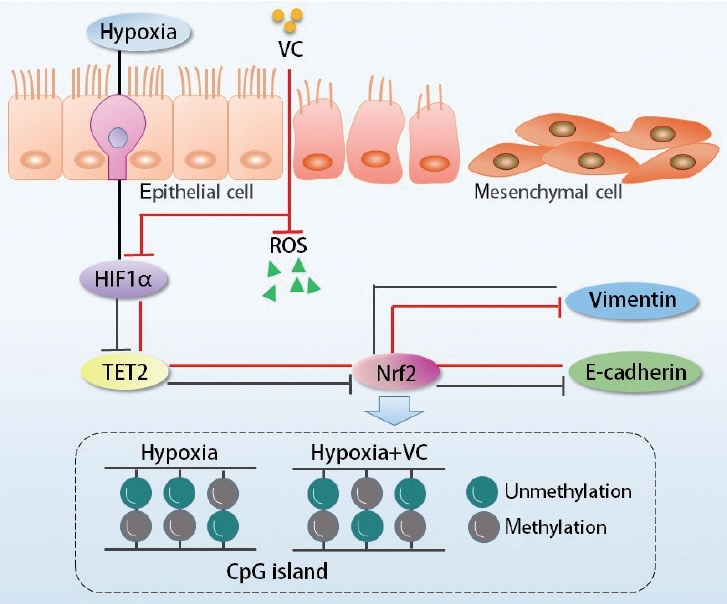
. Hypoxia-inducible factor 1α (HIF1α) and Tet methylcytosine dioxygenase 2 (TET2) have been reported to mediate nasal polypogenesis through the epithelial-to-mesenchymal transition (EMT). Additionally, HIF1α can regulate the expression and function of TET2. However, the precise mechanism of how TET2 regulates the EMT through HIF1α mediation in nasal epithelial cells is still poorly understood.
Methods
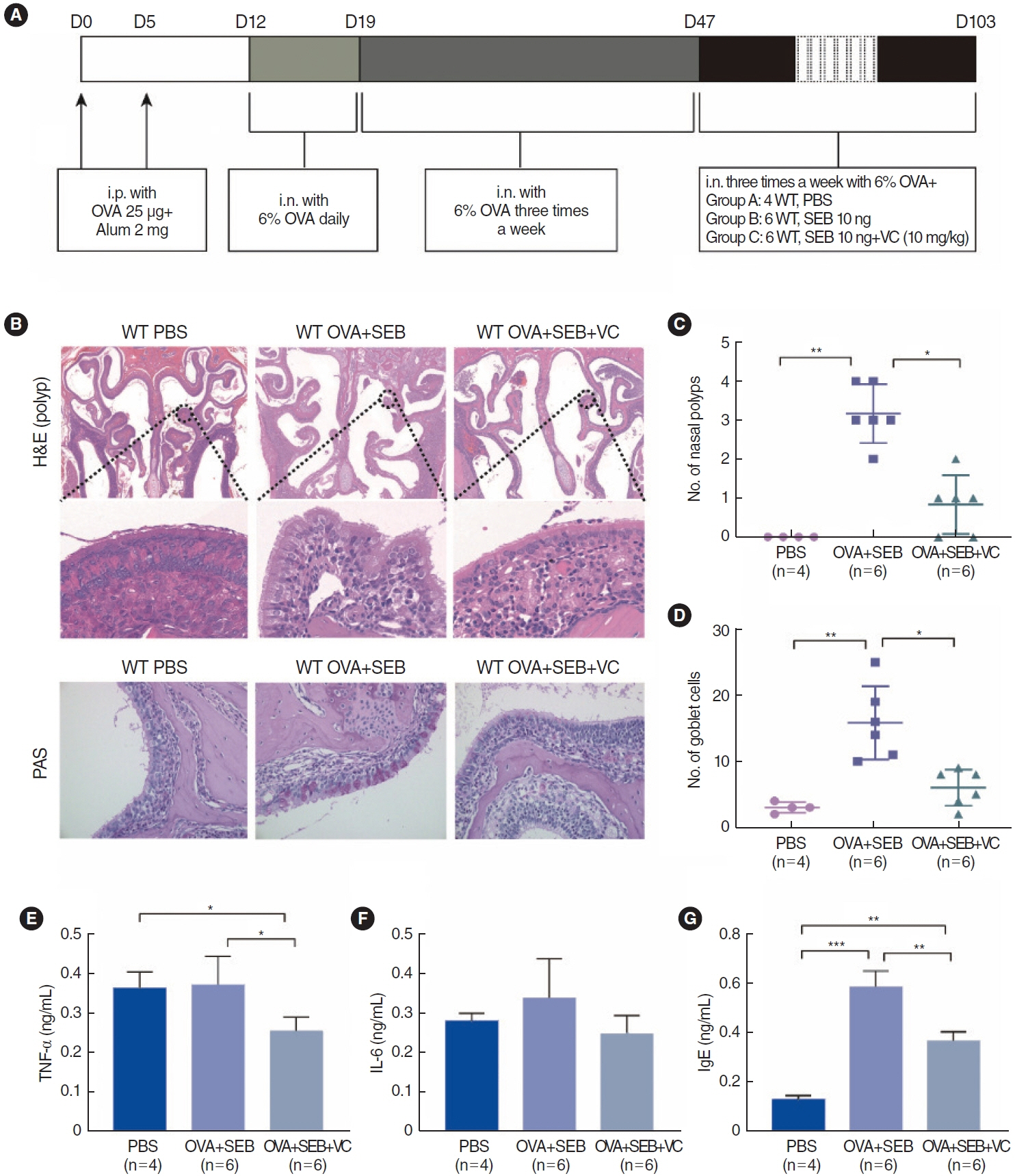
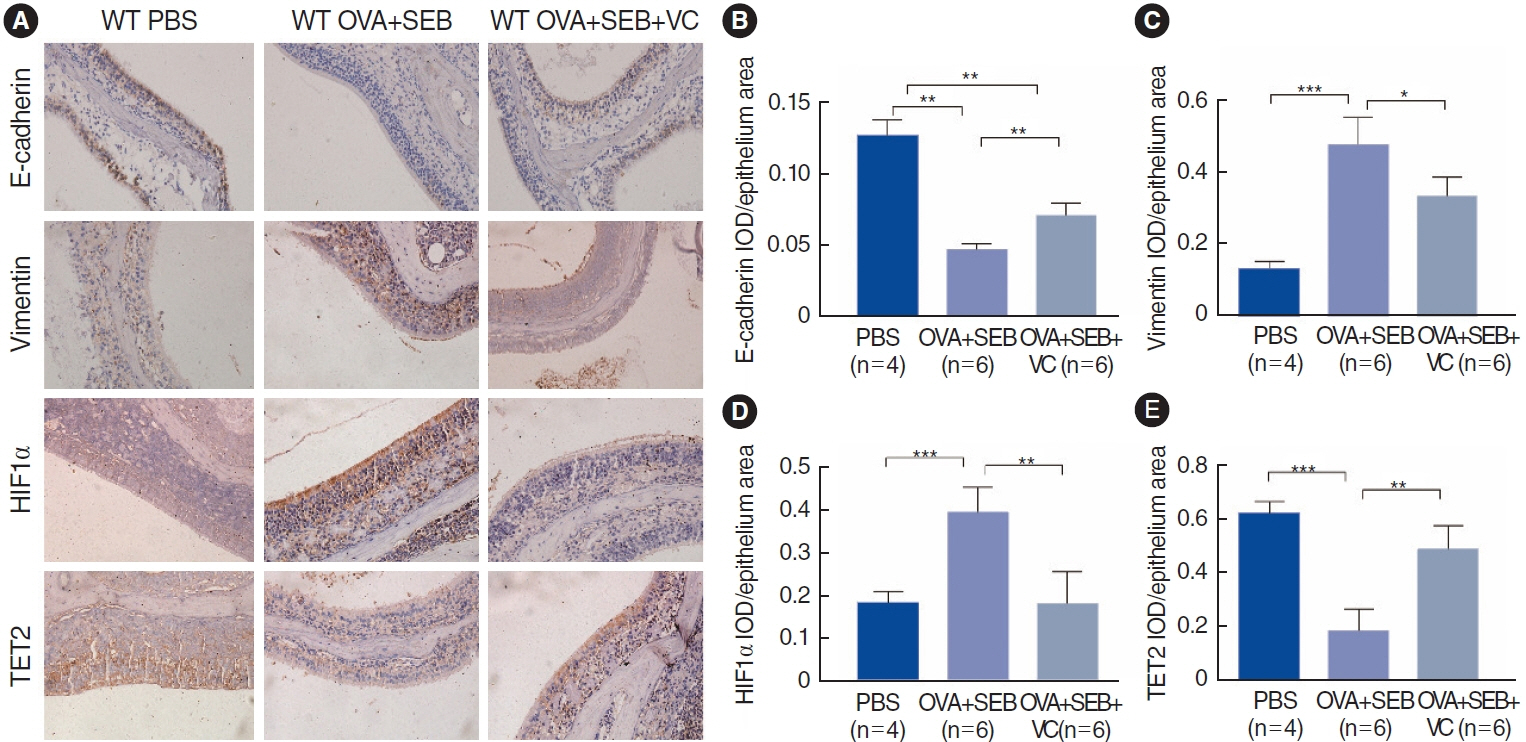
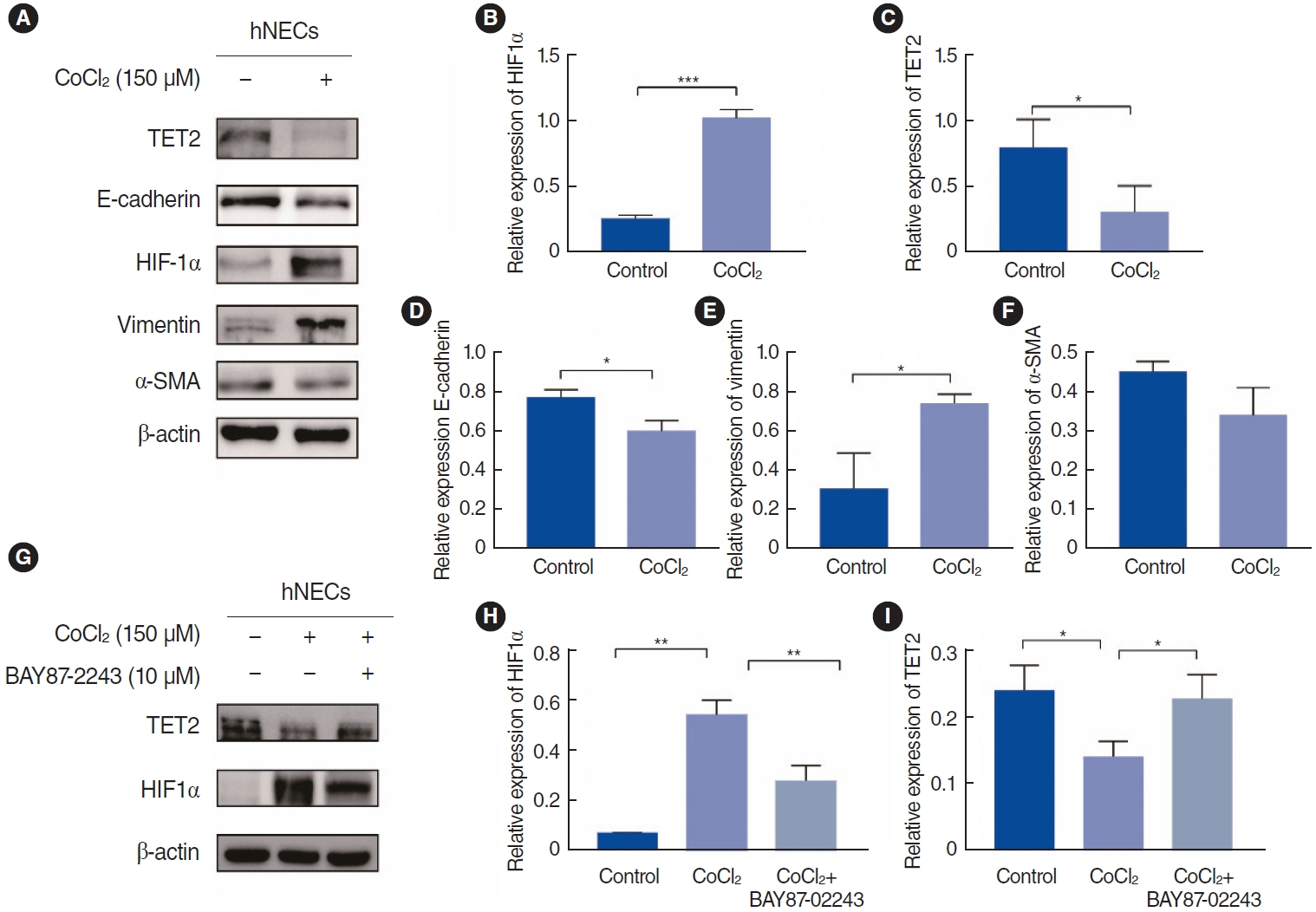
. Nasal tissue samples were collected from patients with chronic rhinosinusitis (CRS) with nasal polyps (CRSwNP), CRS without nasal polyps (CRSsNP), and controls. The expression of HIF1α and TET2 was detected using Western blotting and immunohistochemistry. EMT markers (E-cadherin and vimentin) were also evaluated by immunohistochemistry. Primary human nasal epithelial cells (hNECs) were stimulated with CoCl2 to mimic hypoxia. Vitamin C (VC), a TET2 non-specific activator, and small interfering RNA (siRNA) transfection of TET2 were used to further determine the role of TET2 in hypoxia-induced EMT. Finally, reactive oxygen species (ROS) and Nrf2 were measured to explore the downstream consequences of TET2 in hypoxic hNECs.
Results
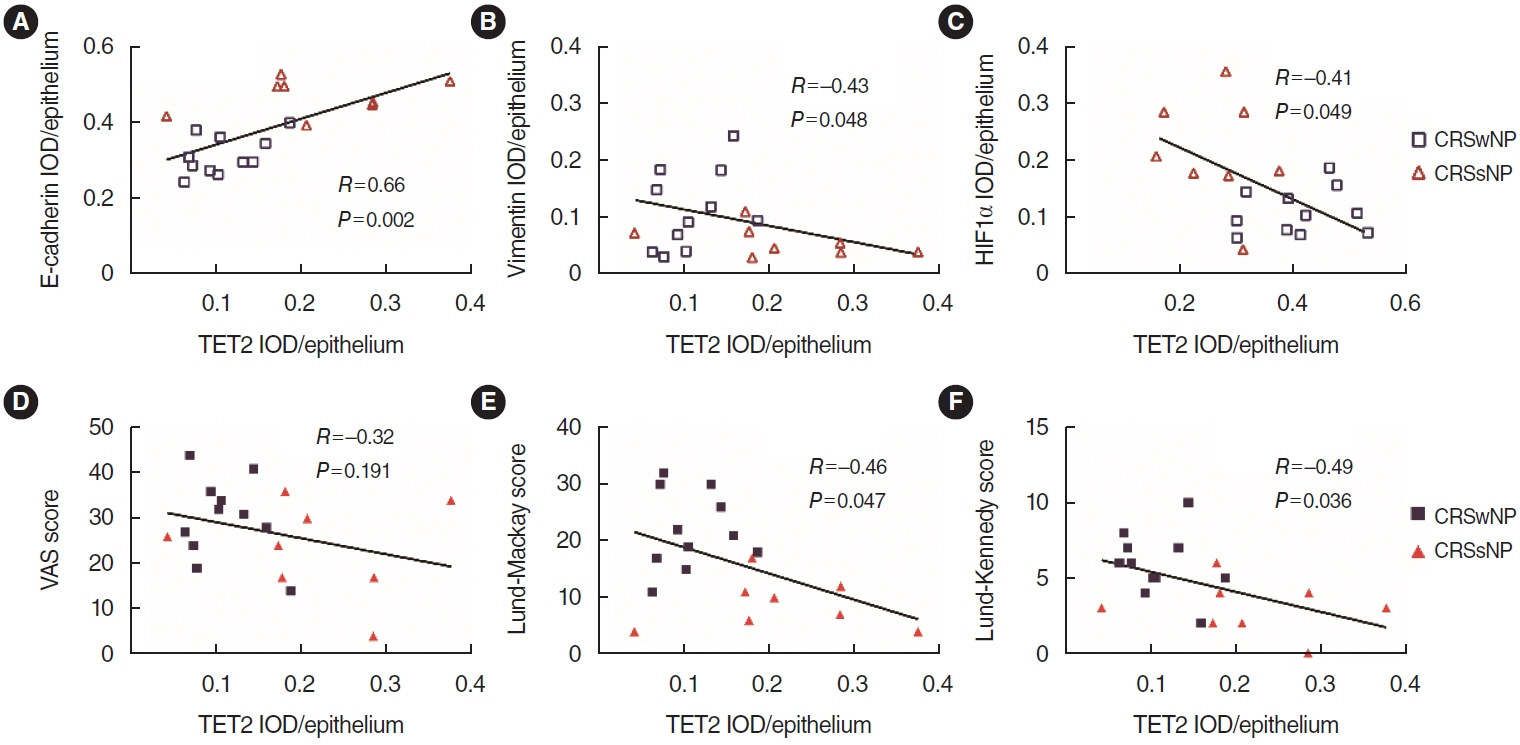
. TET2 levels were lower in the nasal epithelium of CRSwNP patients and were positively correlated with E-cadherin but negatively correlated with vimentin in CRS. However, HIF1α exhibited the opposite pattern and was negatively correlated with TET2 expression. CoCl2-simulated hypoxia led to EMT and increased HIF1α in hNECs in vitro, with simultaneous downregulation of TET2 expression. Addition of VC activated TET2 expression in hNECs, but inhibited EMT and HIF1α expression. Furthermore, siRNA knockdown of TET2 contributed to the EMT in CoCl2-simulated hNECs despite the addition of VC. Finally, TET2 regulated the EMT in hypoxic hNECs through Nrf2 expression and ROS generation.
Conclusion
. TET2 was negatively correlated with HIF1α and EMT in vivo. TET2 was downregulated by HIF1α, resulting in the EMT in CoCl2-hypoxic hNECs via regulation of oxidative stress in vitro. Hence, TET2 might provide a new therapeutic approach for CRSwNP
Figure
Reference
-
1. Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020; Feb. 58(Suppl S29):1–464.2. Hupin C, Gohy S, Bouzin C, Lecocq M, Polette M, Pilette C. Features of mesenchymal transition in the airway epithelium from chronic rhinosinusitis. Allergy. 2014; Nov. 69(11):1540–9.3. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelialmesenchymal transition. Nat Rev Mol Cell Biol. 2014; Mar. 15(3):178–96.4. Lee M, Kim DW, Yoon H, So D, Khalmuratova R, Rhee CS, et al. Sirtuin 1 attenuates nasal polypogenesis by suppressing epithelial-tomesenchymal transition. J Allergy Clin Immunol. 2016; Jan. 137(1):87–98.5. Konnecke M, Burmeister M, Pries R, Boscke R, Bruchhage KL, Ungefroren H, et al. Epithelial-mesenchymal transition in chronic rhinosinusitis: differences revealed between epithelial cells from nasal polyps and inferior turbinates. Arch Immunol Ther Exp (Warsz). 2017; Apr. 65(2):157–73.6. Wu X, Zhang Y. TET-mediated active DNA demethylation: mechanism, function and beyond. Nat Rev Genet. 2017; Sep. 18(9):517–34.7. Hu X, Zhang L, Mao SQ, Li Z, Chen J, Zhang RR, et al. Tet and TDG mediate DNA demethylation essential for mesenchymal-to-epithelial transition in somatic cell reprogramming. Cell Stem Cell. 2014; Apr. 14(4):512–22.8. Ko M, An J, Pastor WA, Koralov SB, Rajewsky K, Rao A. TET proteins and 5-methylcytosine oxidation in hematological cancers. Immunol Rev. 2015; Jan. 263(1):6–21.9. Pastor WA, Aravind L, Rao A. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat Rev Mol Cell Biol. 2013; Jun. 14(6):341–56.10. Jiang S. Tet2 at the interface between cancer and immunity. Commun Biol. 2020; Nov. 3(1):667.11. Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016; Apr. 352(6282):175–80.12. Shin HW, Cho K, Kim DW, Han DH, Khalmuratova R, Kim SW, et al. Hypoxia-inducible factor 1 mediates nasal polypogenesis by inducing epithelial-to-mesenchymal transition. Am J Respir Crit Care Med. 2012; May. 185(9):944–54.13. Lin SK, Shun CT, Kok SH, Wang CC, Hsiao TY, Liu CM. Hypoxia-stimulated vascular endothelial growth factor production in human nasal polyp fibroblasts: effect of epigallocatechin-3-gallate on hypoxia-inducible factor-1 alpha synthesis. Arch Otolaryngol Head Neck Surg. 2008; May. 134(5):522–7.14. Min HJ, Kim JH, Yoo JE, Oh JH, Kim KS, Yoon JH, et al. ROS-dependent HMGB1 secretion upregulates IL-8 in upper airway epithelial cells under hypoxic condition. Mucosal Immunol. 2017; May. 10(3):685–94.15. Min HJ, Choe JW, Kim KS, Yoon JH, Kim CH. High-mobility group box 1 protein induces epithelialmesenchymal transition in upper airway epithelial cells. Rhinology. 2020; Oct. 58(5):495–505.16. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012: a summary for otorhinolaryngologists. Rhinology. 2012; Mar. 50(1):1–12.17. Zeng LH, Wang QM, Feng LY, Ke YD, Xu QZ, Wei AY, et al. High-dose vitamin C suppresses the invasion and metastasis of breast cancer cells via inhibiting epithelial-mesenchymal transition. Onco Targets Ther. 2019; Sep. 12:7405–13.18. Shin JH, Kim KM, Jeong JU, Shin JM, Kang JH, Bang K, et al. Nrf2- heme oxygenase-1 attenuates high-glucose-induced epithelial-tomesenchymal transition of renal tubule cells by inhibiting ROS-mediated PI3K/Akt/GSK-3β signaling. J Diabetes Res. 2019; Aug. 2019:2510105.19. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019; Feb. 20(2):69–84.20. Gagliardo R, Bucchieri F, Montalbano AM, Albano GD, Gras D, Fucarino A, et al. Airway epithelial dysfunction and mesenchymal transition in chronic obstructive pulmonary disease: role of Oct-4. Life Sci. 2022; Jan. 288:120177.21. Tan ML, Huang WJ, Wang Y, Liu L, Pan Y, Li JJ, et al. Integrin-β4 regulates the dynamic changes of phenotypic characteristics in association with epithelial-mesenchymal transition (EMT) and RhoA activity in airway epithelial cells during injury and repair. Int J Biol Sci. 2022; Jan. 18(3):1254–70.22. Lee M, Lim S, Kim YS, Khalmuratova R, Shin SH, Kim I, et al. DEPinduced ZEB2 promotes nasal polyp formation via epithelial-to-mesenchymal transition. J Allergy Clin Immunol. 2022; Jan. 149(1):340–57.23. Zhong B, Seah JJ, Liu F, Ba L, Du J, Wang Y. The role of hypoxia in the pathophysiology of chronic rhinosinusitis. Allergy. 2022; Nov. 77(11):3217–32.24. Sarkar D, Leung EY, Baguley BC, Finlay GJ, Askarian-Amiri ME. Epigenetic regulation in human melanoma: past and future. Epigenetics. 2015; 10(2):103–21.25. Ciesielski P, Jozwiak P, Forma E, Krzeslak A. TET3- and OGT-dependent expression of genes involved in epithelial-mesenchymal transition in endometrial cancer. Int J Mol Sci. 2021; Dec. 22(24):13239.26. Ye Z, Li J, Han X, Hou H, Chen H, Zheng X, et al. TET3 inhibits TGFβ1-induced epithelial-mesenchymal transition by demethylating miR30d precursor gene in ovarian cancer cells. J Exp Clin Cancer Res. 2016; May. 35:72.27. Thienpont B, Steinbacher J, Zhao H, D’Anna F, Kuchnio A, Ploumakis A, et al. Tumour hypoxia causes DNA hypermethylation by reducing TET activity. Nature. 2016; Sep. 537(7618):63–8.28. Kim JY, Kim DK, Yu MS, Cha MJ, Yu SL, Kang J. Role of epigenetics in the pathogenesis of chronic rhinosinusitis with nasal polyps. Mol Med Rep. 2018; Jan. 17(1):1219–27.29. Shen Q, Zhang Q, Shi Y, Shi Q, Jiang Y, Gu Y, et al. Tet2 promotes pathogen infection-induced myelopoiesis through mRNA oxidation. Nature. 2018; Feb. 554(7690):123–7.30. Zhang Q, Zhao K, Shen Q, Han Y, Gu Y, Li X, et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature. 2015; Sep. 525(7569):389–93.31. Zeng Z, Li T, Liu X, Ma Y, Luo L, Wang Z, et al. DNA dioxygenases TET2 deficiency promotes cigarette smoke induced chronic obstructive pulmonary disease by inducing ferroptosis of lung epithelial cell. Redox Biol. 2023; Nov. 67:102916.32. Tan L, Fu L, Zheng L, Fan W, Tan H, Tao Z, et al. TET2 regulates 5-hydroxymethylcytosine signature and CD4+ T-cell balance in allergic rhinitis. Allergy Asthma Immunol Res. 2022; Mar. 14(2):254–72.33. Tan L, Qiu T, Xiang R, Cao C, Deng Y, Tao Z, et al. Down-regulation of Tet2 is associated with Foxp3 TSDR hypermethylation in regulatory T cell of allergic rhinitis. Life Sci. 2020; Jan. 241:117101.34. Zhang X, Li S, He J, Jin Y, Zhang R, Dong W, et al. TET2 suppresses VHL deficiency-driven clear cell renal cell carcinoma by inhibiting HIF signaling. Cancer Res. 2022; Jun. 82(11):2097–109.35. Fischer AP, Miles SL. Silencing HIF-1α induces TET2 expression and augments ascorbic acid induced 5-hydroxymethylation of DNA in human metastatic melanoma cells. Biochem Biophys Res Commun. 2017; Aug. 490(2):176–81.36. Wan F, Tang YW, Tang XL, Li YY, Yang RC. TET2 mediated demethylation is involved in the protective effect of triptolide on podocytes. Am J Transl Res. 2021; Mar. 13(3):1233–44.37. Gong F, Guo Y, Niu Y, Jin J, Zhang X, Shi X, et al. Epigenetic silencing of TET2 and TET3 induces an EMT-like process in melanoma. Oncotarget. 2017; Jan. 8(1):315–28.38. Kim JH, Hwang S, Lee JH, Im SS, Son J. Vitamin C suppresses pancreatic carcinogenesis through the inhibition of both glucose metabolism and Wnt signaling. Int J Mol Sci. 2022; Oct. 23(20):12249.39. Lin H, Ba G, Tang R, Li M, Li Z, Li D, et al. Increased expression of TXNIP facilitates oxidative stress in nasal epithelial cells of patients with chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2021; Sep. 35(5):607–14.40. Zheng K, Hao J, Xiao L, Wang M, Zhao Y, Fan D, et al. Expression of nicotinamide adenine dinucleotide phosphate oxidase in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2020; May. 10(5):646–55.41. Ramanathan M Jr, Tharakan A, Sidhaye VK, Lane AP, Biswal S, London NR Jr. Disruption of sinonasal epithelial Nrf2 enhances susceptibility to rhinosinusitis in a mouse model. Laryngoscope. 2021; Apr. 131(4):713–9.42. Tharakan A, Halderman AA, Lane AP, Biswal S, Ramanathan M Jr. Reversal of cigarette smoke extract-induced sinonasal epithelial cell barrier dysfunction through Nrf2 Activation. Int Forum Allergy Rhinol. 2016; Nov. 6(11):1145–50.43. Qin D, Liu P, Zhou H, Jin J, Gong W, Liu K, et al. TIM-4 in macrophages contributes to nasal polyp formation through the TGF-β1- mediated epithelial to mesenchymal transition in nasal epithelial cells. Front Immunol. 2022; Aug. 13:941608.44. Liu P, Qin D, Deng Z, Tong X, Liu K, Fan W, et al. TET2 deficiency exacerbates nasal polypogenesis by inducing epithelial-to-mesenchymal transition. Allergy. 2022; Nov. 77(11):3452–5.45. Wee JH, Ko YK, Khalmuratova R, Shin HW, Kim DW, Rhee CS. Effect of lipopolysaccharide and polyinosinic:polycytidylic acid in a murine model of nasal polyp. Sci Rep. 2021; Jan. 11(1):1021.46. Bae JS, Ryu G, Kim JH, Kim EH, Rhee YH, Chung YJ, et al. Effects of Wnt signaling on epithelial to mesenchymal transition in chronic rhinosinusitis with nasal polyp. Thorax. 2020; Nov. 75(11):982–93.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ACY-241, a histone deacetylase 6 inhibitor, suppresses the epithelial–mesenchymal transition in lung cancer cells by downregulating hypoxia-inducible factor-1 alpha
- Anti-Tumor Effect of IDF-11774, an Inhibitor of Hypoxia-Inducible Factor-1, on Melanoma
- Wheatgrass extract inhibits hypoxia-inducible factor-1-mediated epithelial-mesenchymal transition in A549 cells
- Vitexin Inhibits Gastric Cancer Growth and Metastasis through HMGB1-mediated Inactivation of the PI3K/ AKT/mTOR/HIF-1α Signaling Pathway
- Myocardin Reverses Hypoxia-Inducible Factor-1α Mediated Phenotypic Modulation of Corpus Cavernosum Smooth Muscle Cells in Hypoxia Induced by Cobalt Chloride