Clin Endosc.
2024 May;57(3):393-401. 10.5946/ce.2023.068.
The role of needle-based confocal laser endomicroscopy in the diagnosis of pancreatic neuroendocrine tumors
- Affiliations
-
- 1Department of Gastroenterology, Aichi Cancer Center Hospital, Nagoya, Japan
- KMID: 2555901
- DOI: http://doi.org/10.5946/ce.2023.068
Abstract
- Background/Aims
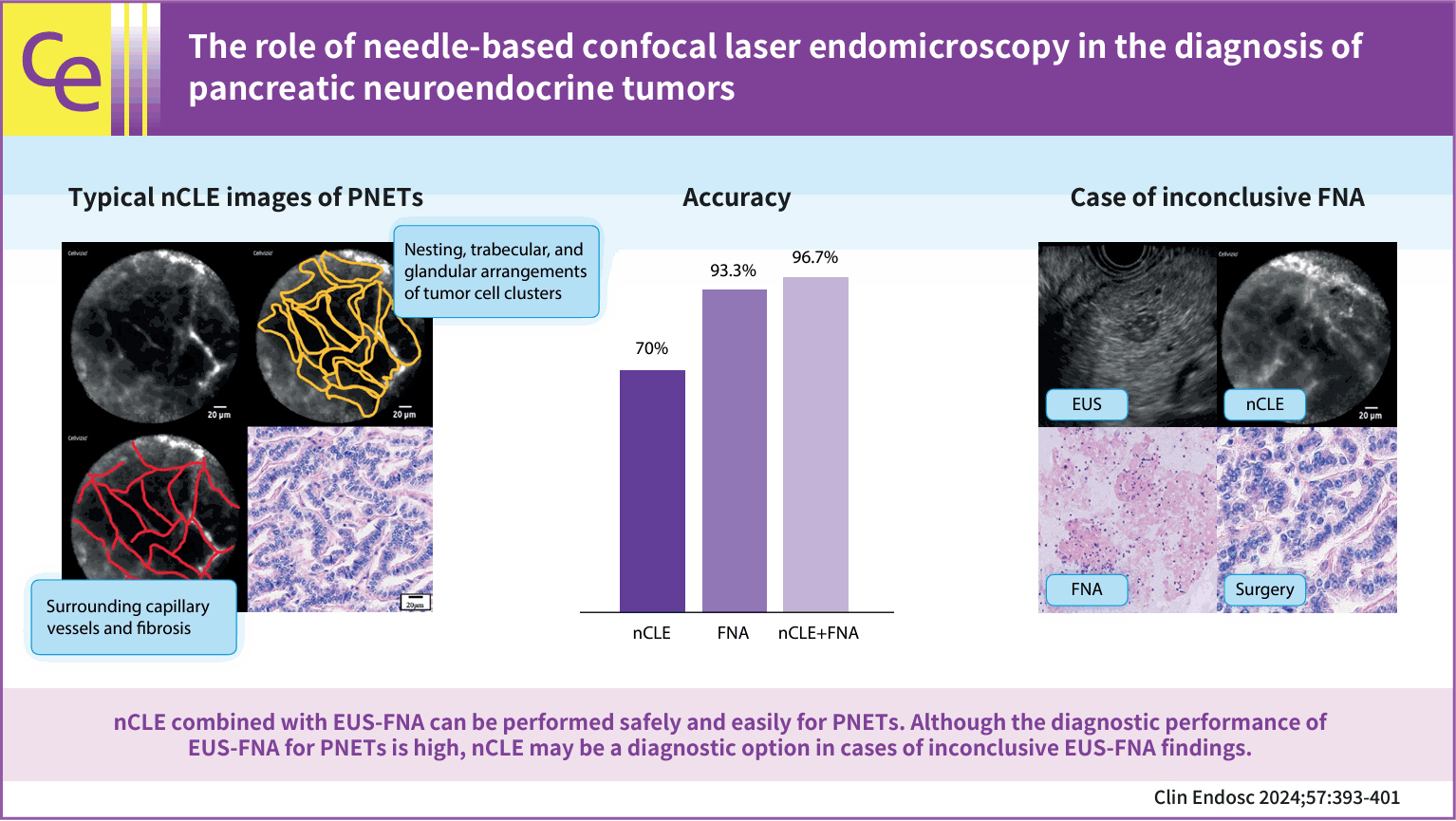
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is a highly accurate method for diagnosing pancreatic neuroendocrine tumors (PNETs); however, some PNETs are difficult to diagnose. Recently, the efficacy of needle-based confocal laser endomicroscopy (nCLE) in diagnosing solid pancreatic masses has been reported. However, the efficacy of nCLE in the diagnosis of PNETs remains unknown and only a small number of cases have been reported. Hence, this study aimed to evaluate the efficacy of nCLE in the diagnosis of PNETs.
Methods
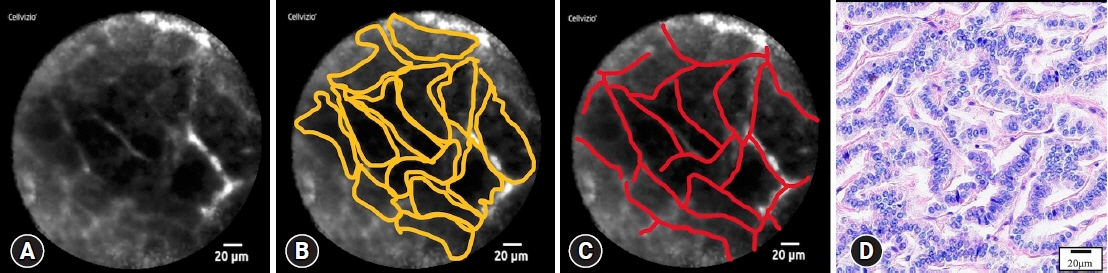
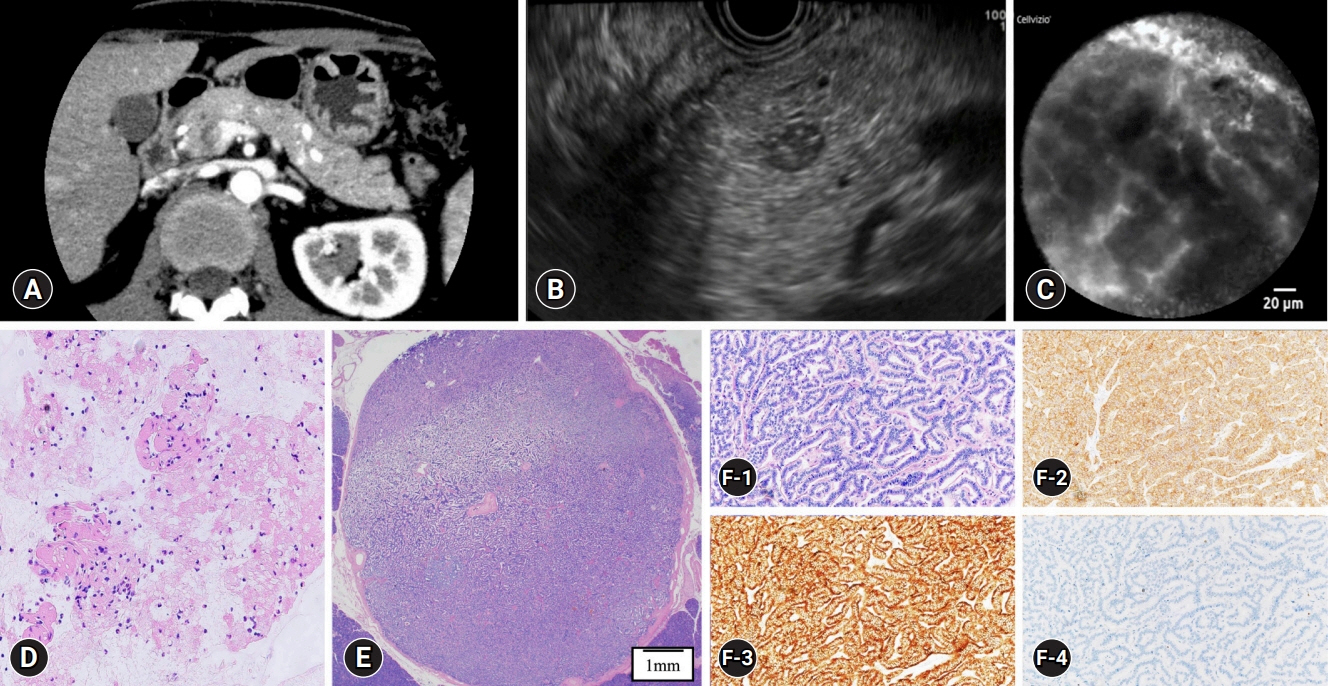
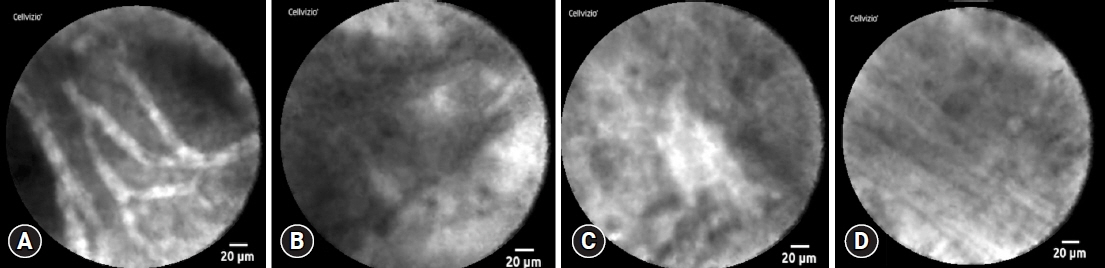
This single-center retrospective study evaluated 30 consecutive patients with suspected PNETs on contrast-enhanced computed tomography, who consented to nCLE combined with EUS-FNA and were diagnosed using EUS-FNA or surgical resection. The diagnostic criteria for PNETs using nCLE were based on the nesting and trabecular and glandular arrangement of tumor cell clusters surrounded by capillary vessels and fibrosis, as reported in previous studies.
Results
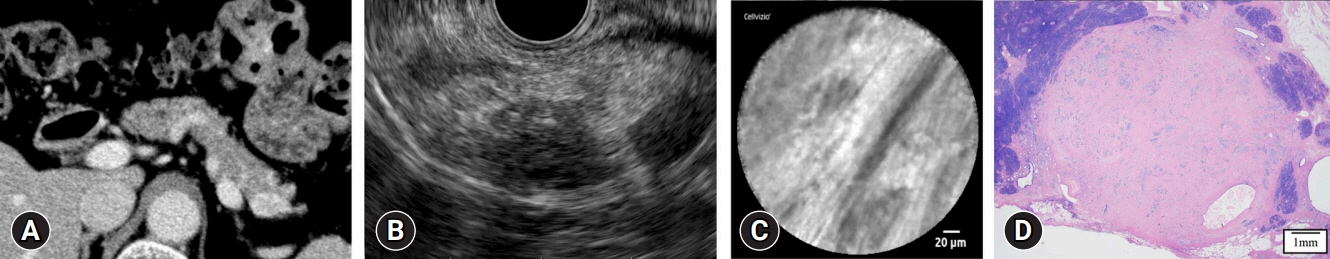
The diagnosis using nCLE was classified into three categories: misdiagnosis in three cases (10%), non-diagnostic in six cases (20%), and diagnostic in 21 cases (70%). nCLE was able to diagnose PNET in one of the two cases with inconclusive EUS-FNA.
Conclusions
Although further development of the resolution and optimization of the diagnostic criteria are required, nCLE may constitute a useful diagnostic option in cases of inconclusive EUS-FNA for PNETs.
Keyword
Figure
Reference
-
1. Ishii T, Katanuma A, Toyonaga H, et al. Role of endoscopic ultrasound in the diagnosis of pancreatic neuroendocrine neoplasms. Diagnostics (Basel). 2021; 11:316.
Article2. Ito T, Hijioka S, Masui T, et al. Advances in the diagnosis and treatment of pancreatic neuroendocrine neoplasms in Japan. J Gastroenterol. 2017; 52:9–18.
Article3. Krishna SG, Bhattacharya A, Li F, et al. Diagnostic differentiation of pancreatic neuroendocrine tumor from other neoplastic solid pancreatic lesions during endoscopic ultrasound-guided fine-needle aspiration. Pancreas. 2016; 45:394–400.
Article4. Heidsma CM, Tsilimigras DI, Rocha F, et al. Clinical relevance of performing endoscopic ultrasound-guided fine-needle biopsy for pancreatic neuroendocrine tumors less than 2 cm. J Surg Oncol. 2020; 122:1393–1400.
Article5. Hedenström P. The best approach for sampling of pancreatic neuroendocrine tumors: EUS-FNA or EUS-FNB? Endosc Int Open. 2019; 7:E1400–E1402.6. Eusebi LH, Thorburn D, Toumpanakis C, et al. Endoscopic ultrasound-guided fine-needle aspiration vs fine-needle biopsy for the diagnosis of pancreatic neuroendocrine tumors. Endosc Int Open. 2019; 7:E1393–E1399.
Article7. Witt BL, Factor RE, Chadwick BE, et al. Evaluation of the SharkCore® needle for EUS-guided core biopsy of pancreatic neuroendocrine tumors. Endosc Ultrasound. 2018; 7:323–328.
Article8. Leeds JS, Nayar MK, Bekkali NL, et al. Endoscopic ultrasound-guided fine-needle biopsy is superior to fine-needle aspiration in assessing pancreatic neuroendocrine tumors. Endosc Int Open. 2019; 7:E1281–E1287.
Article9. Di Leo M, Poliani L, Rahal D, et al. Pancreatic neuroendocrine tumours: the role of endoscopic ultrasound biopsy in diagnosis and grading based on the WHO 2017 classification. Dig Dis. 2019; 37:325–333.
Article10. Kamata K, Ashida R, Yasukawa S, et al. Histological diagnosis and grading of pancreatic neuroendocrine tumor by endoscopic ultrasound-guided fine needle biopsy using a 25-gauge needle with a core trap: a multicenter prospective trial. Pancreatology. 2020; 20:1428–1433.
Article11. Hijioka S, Hara K, Mizuno N, et al. Diagnostic performance and factors influencing the accuracy of EUS-FNA of pancreatic neuroendocrine neoplasms. J Gastroenterol. 2017; 52:264.
Article12. Konda VJ, Meining A, Jamil LH, et al. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013; 45:1006–1013.
Article13. Haba S, Yamao K, Bhatia V, et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J Gastroenterol. 2013; 48:973–981.
Article14. Kongkam P, Pittayanon R, Sampatanukul P, et al. Endoscopic ultrasound-guided needle-based confocal laser endomicroscopy for diagnosis of solid pancreatic lesions (ENES): a pilot study. Endosc Int Open. 2016; 4:E17–E23.
Article15. Giovannini M, Caillol F, Monges G, et al. Endoscopic ultrasound-guided needle-based confocal laser endomicroscopy in solid pancreatic masses. Endoscopy. 2016; 48:892–898.
Article16. Karstensen JG, Cârţână T, Constantinescu C, et al. Endoscopic ultrasound guided needle-based confocal laser endomicroscopy in solid pancreatic masses: a prospective validation study. Endosc Int Open. 2018; 6:E78–E85.17. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010; 71:446–454.
Article18. McCall CM, Shi C, Klein AP, et al. Serotonin expression in pancreatic neuroendocrine tumors correlates with a trabecular histologic pattern and large duct involvement. Hum Pathol. 2012; 43:1169–1176.
Article19. Nakai Y, Iwashita T, Park DH, et al. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015; 81:1204–1214.
Article20. Okuno N, Hara K, Obata M. Novel method of diagnosing solid pseudopapillary neoplasms of the pancreas: Needle-based confocal laser endomicroscopy. Dig Endosc. 2019; 31:461.
Article21. Thoeni RF, Mueller-Lisse UG, Chan R, et al. Detection of small, functional islet cell tumors in the pancreas: selection of MR imaging sequences for optimal sensitivity. Radiology. 2000; 214:483–490.
Article22. Lewis RB, Lattin GE Jr, Paal E. Pancreatic endocrine tumors: radiologic-clinicopathologic correlation. Radiographics. 2010; 30:1445–1464.
Article23. Krishna SG, Brugge WR, Dewitt JM, et al. Needle-based confocal laser endomicroscopy for the diagnosis of pancreatic cystic lesions: an international external interobserver and intraobserver study (with videos). Gastrointest Endosc. 2017; 86:644–654.
Article24. Haq I, Krishna SG, Patel B, et al. The impact of repeating endosonography with confocal endomicroscopy for the diagnosis of cystic neuroendocrine tumor. Case Rep Gastrointest Med. 2019; 2019:5187874.
Article25. Sharma P, Meining AR, Coron E, et al. Real-time increased detection of neoplastic tissue in Barrett's esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2011; 74:465–472.
Article26. Bertani H, Frazzoni M, Dabizzi E, et al. Improved detection of incident dysplasia by probe-based confocal laser endomicroscopy in a Barrett's esophagus surveillance program. Dig Dis Sci. 2013; 58:188–193.
Article27. Bok GH, Jeon SR, Cho JY, et al. The accuracy of probe-based confocal endomicroscopy versus conventional endoscopic biopsies for the diagnosis of superficial gastric neoplasia (with videos). Gastrointest Endosc. 2013; 77:899–908.
Article28. Lim LG, Yeoh KG, Srivastava S, et al. Comparison of probe-based confocal endomicroscopy with virtual chromoendoscopy and white-light endoscopy for diagnosis of gastric intestinal metaplasia. Surg Endosc. 2013; 27:4649–4655.
Article29. van den Broek FJ, van Es JA, van Eeden S, et al. Pilot study of probe-based confocal laser endomicroscopy during colonoscopic surveillance of patients with longstanding ulcerative colitis. Endoscopy. 2011; 43:116–122.
Article30. Rispo A, Castiglione F, Staibano S, et al. Diagnostic accuracy of confocal laser endomicroscopy in diagnosing dysplasia in patients affected by long-standing ulcerative colitis. World J Gastrointest Endosc. 2012; 4:414–420.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Needle-Based Confocal Laser Endomicroscopy in the Evaluation of Pancreatic Cystic Lesions: A Systematic Review
- Endoscopic ultrasound-guided needle-based confocal laser endomicroscopy for diagnosis of solid pancreatic lesions
- Endoscopic ultrasound-guided needle-based confocal laser endomicroscopy for pancreatic cystic lesions: current status and future prospects
- A Review of Probe-Based Confocal Laser Endomicroscopy for Pancreaticobiliary Disease
- Endoscopic Ultrasound-Based Evaluation of Pancreatic Cysts: New Invasive Modalities