Brain Tumor Res Treat.
2024 Apr;12(2):125-131. 10.14791/btrt.2024.0008.
Spontaneous Regression of a Large Vestibular Schwannoma: Is Nonoperative Management Reasonable?
- Affiliations
-
- 1Division of Neurosurgery, National University Hospital, Singapore
- 2Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore
- 3Division of Neurosurgery, Ng Teng Fong General Hospital, Singapore
- KMID: 2555872
- DOI: http://doi.org/10.14791/btrt.2024.0008
Abstract
- Vestibular schwannomas (VSs) are the most common cerebellopontine tumors. The natural history of smaller-sized VSs (<30 mm) has been well-studied, leading to the recommendation of a “watch and wait” approach. However, large VSs (>30 mm) have not been extensively studied, mainly because of their rarity. As such, most patients are conventionally offered surgery which carries a significant risk of neurological morbidity. Here, we report a case of a giant VS (>40 mm) in a 30-year-old man who regressed spontaneously. He was lost to follow-up for 18 years and, upon re-presentation, the symptomatology drastically improved and repeat imaging demonstrated a marked reduction in tumor size. Referring to similar cases in other studies, we postulate that most large and giant VSs undergo a phase of growth and stasis, followed by regression due to shifts in the balance between tumorigenic and regressive factors. Taken together with emerging molecular data, further studies are required to better understand the history of large and giant VSs to shape more personalized treatment options. This potentially includes non-operative management as a tenable option.
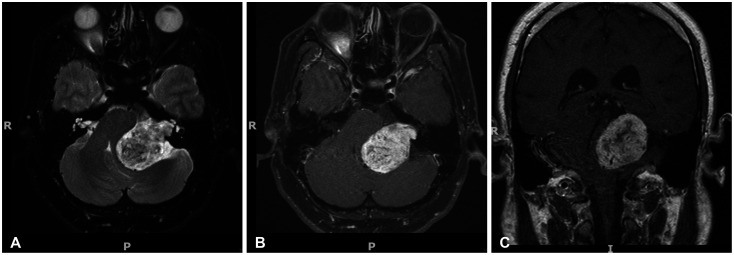
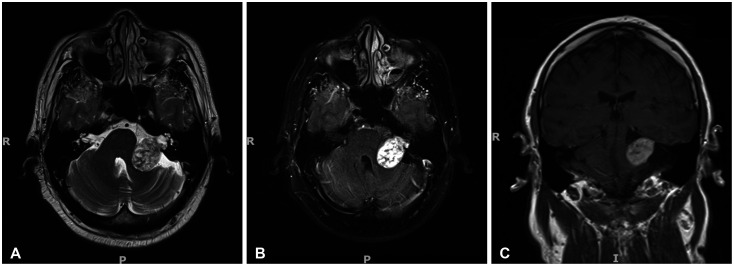
Figure
Reference
-
1. Carlson ML, Link MJ. Vestibular schwannomas. N Engl J Med. 2021; 384:1335–1348. PMID: 33826821.2. Stivaros SM, Stemmer-Rachamimov AO, Alston R, Plotkin SR, Nadol JB, Quesnel A, et al. Multiple synchronous sites of origin of vestibular schwannomas in neurofibromatosis type 2. J Med Genet. 2015; 52:557–562. PMID: 26104281.3. Dewan R, Pemov A, Kim HJ, Morgan KL, Vasquez RA, Chittiboina P, et al. Evidence of polyclonality in neurofibromatosis type 2-associated multilobulated vestibular schwannomas. Neuro Oncol. 2015; 17:566–573. PMID: 25452392.4. Carlson ML, Glasgow AE, Grossardt BR, Habermann EB, Link MJ. Does where you live influence how your vestibular schwannoma is managed? Examining geographical differences in vestibular schwannoma treatment across the United States. J Neurooncol. 2016; 129:269–279. PMID: 27334903.5. Starnoni D, Giammattei L, Cossu G, Link MJ, Roche PH, Chacko AG, et al. Surgical management for large vestibular schwannomas: a systematic review, meta-analysis, and consensus statement on behalf of the EANS skull base section. Acta Neurochir (Wien). 2020; 162:2595–2617. PMID: 32728903.6. Müller S, Arnolds J, van Oosterhout A. Decision-making of vestibular schwannoma patients. Acta Neurochir (Wien). 2010; 152:973–984. PMID: 20169371.7. Marinelli JP, Lohse CM, Grossardt BR, Lane JI, Carlson ML. Rising incidence of sporadic vestibular schwannoma: true biological shift versus simply greater detection. Otol Neurotol. 2020; 41:813–847. PMID: 32150020.8. Evans DGR. Neurofibromatosis 2 [Bilateral acoustic neurofibromatosis, central neurofibromatosis, NF2, neurofibromatosis type II]. Genet Med. 2009; 11:599–610. PMID: 19652604.9. Stangerup SE, Caye-Thomasen P. Epidemiology and natural history of vestibular schwannomas. Otolaryngol Clin North Am. 2012; 45:257–268. PMID: 22483814.10. Reznitsky M, Petersen MMBS, West N, Stangerup SE, Cayé-Thomasen P. Epidemiology of vestibular schwannomas–prospective 40-year data from an unselected national cohort. Clin Epidemiol. 2019; 11:981–986. PMID: 31807080.11. Samii M, Gerganov VM, Samii A. Functional outcome after complete surgical removal of giant vestibular schwannomas. J Neurosurg. 2010; 112:860–867. PMID: 19663543.12. Artz JC, Timmer FC, Mulder JJ, Cremers CW, Graamans K. Predictors of future growth of sporadic vestibular schwannomas obtained by history and radiologic assessment of the tumor. Eur Arch Otorhinolaryngol. 2009; 266:641–646. PMID: 18704473.13. Foley RW, Shirazi S, Maweni RM, Walsh K, McConn Walsh R, Javadpour M, et al. Signs and symptoms of acoustic neuroma at initial presentation: an exploratory analysis. Cureus. 2017; 9:e1846. PMID: 29348989.14. Huang X, Xu J, Xu M, Zhou LF, Zhang R, Lang L, et al. Clinical features of intracranial vestibular schwannomas. Oncol Lett. 2013; 5:57–62. PMID: 23255894.15. Gréant E, Van de Heyning P, Ihtijarevic B, Topsakal V, Menovsky T, Van Rompaey V. Sporadic vestibular schwannoma: correlation between tumour size, hearing levels, age and radiologic features in 384 patients. B-ENT. 2020; 16:97–102.16. Nicoucar K, Momjian S, Vader JP, De Tribolet N. Surgery for large vestibular schwannomas: how patients and surgeons perceive quality of life. J Neurosurg. 2006; 105:205–212. PMID: 17219824.17. Daniel RT, Tuleasca C, George M, Pralong E, Schiappacasse L, Zeverino M, et al. Preserving normal facial nerve function and improving hearing outcome in large vestibular schwannomas with a combined approach: planned subtotal resection followed by gamma knife radiosurgery. Acta Neurochir (Wien). 2017; 159:1197–1211. PMID: 28516364.18. Carlson ML, Tveiten OV, Driscoll CL, Goplen FK, Neff BA, Pollock BE, et al. Long-term quality of life in patients with vestibular schwannoma: an international multicenter cross-sectional study comparing microsurgery, stereotactic radiosurgery, observation, and nontumor controls. J Neurosurg. 2015; 122:833–842. PMID: 25555165.19. Tuleasca C, Kotecha R, Sahgal A, de Salles A, Fariselli L, Paddick I, et al. Single-fraction radiosurgery outcomes for large vestibular schwannomas in the upfront or post-surgical setting: a systematic review and International Stereotactic Radiosurgery Society (ISRS) practice guidelines. J Neurooncol. 2023; 165:1–20. PMID: 37843727.20. Brown A, Early S, Vasilijic S, Stankovic KM. Sporadic vestibular schwannoma size and location do not correlate with the severity of hearing loss at initial presentation. Front Oncol. 2022; 12:836504. PMID: 35372070.21. Nikolopoulos TP, Fortnum H, O’Donoghue G, Baguley D. Acoustic neuroma growth: a systematic review of the evidence. Otol Neurotol. 2010; 31:478–485. PMID: 20147867.22. Reddy CE, Lewis-Jones HG, Javadpour M, Ryland I, Lesser TH. Conservative management of vestibular schwannomas of 15 to 31 mm intracranial diameter. J Laryngol Otol. 2014; 128:752–758. PMID: 25120176.23. Yamakami I, Uchino Y, Kobayashi E, Yamaura A. Conservative management, gamma-knife radiosurgery, and microsurgery for acoustic neurinomas: a systematic review of outcome and risk of three therapeutic options. Neurol Res. 2003; 25:682–690. PMID: 14579782.24. Bakkouri WE, Kania RE, Guichard JP, Lot G, Herman P, Huy PT. Conservative management of 386 cases of unilateral vestibular schwannoma: tumor growth and consequences for treatment. J Neurosurg. 2009; 110:662–669. PMID: 19099381.25. Paldor I, Chen AS, Kaye AH. Growth rate of vestibular schwannoma. J Clin Neurosci. 2016; 32:1–8. PMID: 27450283.26. Timmer FC, Graamans K. A large vestibular schwannoma that did not grow for 18 years. B-ENT. 2011; 7:305–307. PMID: 22338247.27. Dahamou M, Elfarissi MA, Lhamlili M, Mehfoud I, Khoulali M, Oulali N, et al. Spontaneous regression of solid-cystic vestibular schwannoma: a case report. Surg Neurol Int. 2022; 13:414. PMID: 36324975.28. Patel EJ, Deep NL, Schecht M, Hagiwara M, Roland JT Jr. Tracking spontaneous vestibular schwannoma regression with volumetric measurements. Laryngoscope. 2021; 131:E1647–E1652. PMID: 33103767.29. Tikka T, Yiannakis CP, Stapleton E, Locke R, Crowther JA, Taylor WAS, et al. Spontaneous vestibular schwannoma regression: a case-control study. Otol Neurotol. 2018; 39:e1118–e1124. PMID: 30106843.30. Amoo M, Rawluk D, Mcconn-Walsh R, Javadpour M. The shrinking vestibular schwannoma. Br J Neurosurg. 2023; 37:701–702. PMID: 30829550.31. Tesařová M, Peterková L, Šťastná M, Kolář M, Lacina L, Smetana K Jr, et al. Tumor biology and microenvironment of vestibular schwannoma-relation to tumor growth and hearing loss. Biomedicines. 2022; 11:32. PMID: 36672540.32. Petrilli AM, Fernández-Valle C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene. 2016; 35:537–548. PMID: 25893302.33. Xu M, Wang S, Jiang Y, Wang J, Xiong Y, Dong W, et al. Single-cell RNA-Seq reveals heterogeneity of cell communications between Schwann cells and fibroblasts within vestibular schwannoma microenvironment. Am J Pathol. 2022; 192:1230–1249. PMID: 35750260.34. Lu VM, Ravindran K, Graffeo CS, Perry A, Van Gompel JJ, Daniels DJ, et al. Efficacy and safety of bevacizumab for vestibular schwannoma in neurofibromatosis type 2: a systematic review and meta-analysis of treatment outcomes. J Neurooncol. 2019; 144:239–248. PMID: 31254266.35. Hannan CJ, Lewis D, O’Leary C, Donofrio CA, Evans DG, Roncaroli F, et al. The inflammatory microenvironment in vestibular schwannoma. Neurooncol Adv. 2020; 2:vdaa023. PMID: 32642684.36. Landry AP, Wang JZ, Suppiah S, Zadeh G. Multiplatform molecular analysis of vestibular schwannoma reveals two robust subgroups with distinct microenvironment. J Neurooncol. 2023; 161:491–499. PMID: 36701029.37. Amit M, Xie T, Gleber-Netto FO, Hunt PJ, Mehta GU, Bell D, et al. Distinct immune signature predicts progression of vestibular schwannoma and unveils a possible viral etiology. J Exp Clin Cancer Res. 2022; 41:292. PMID: 36195959.38. Kim BS, Jung TY, Moon KS, Kim IY, Jung S. Meningioma with partial and spontaneous regression of peritumoral edema on long-term follow up. Brain Tumor Res Treat. 2022; 10:275–278. PMID: 36347643.39. Parsa CF, Hoyt CS, Lesser RL, Weinstein JM, Strother CM, Muci-Mendoza R, et al. Spontaneous regression of optic gliomas: thirteen cases documented by serial neuroimaging. Arch Ophthalmol. 2001; 119:516–529. PMID: 11296017.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and Management of Vestibular Schwannoma: Focus on Dizziness
- Hypervascular Vestibular Schwannoma: A Case Report
- A Case of Intralabyrinthine Schwannoma and Literature Review of the Cases Reported Previously in Korea
- Normal pressure hydrocephalus after gamma knife radiosurgery in a patient with vestibular schwannoma
- A Case of inferior vestibular schwannoma which was lately diagnosed due to normal hearing level