J Korean Assoc Oral Maxillofac Surg.
2024 Apr;50(2):94-102. 10.5125/jkaoms.2024.50.2.94.
Elemental characteristics of sialoliths extracted from a patient with recurrent sialolithiasis
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, Dental Research Institute, School of Dentistry, Seoul National University, Seoul, Korea
- KMID: 2555739
- DOI: http://doi.org/10.5125/jkaoms.2024.50.2.94
Abstract
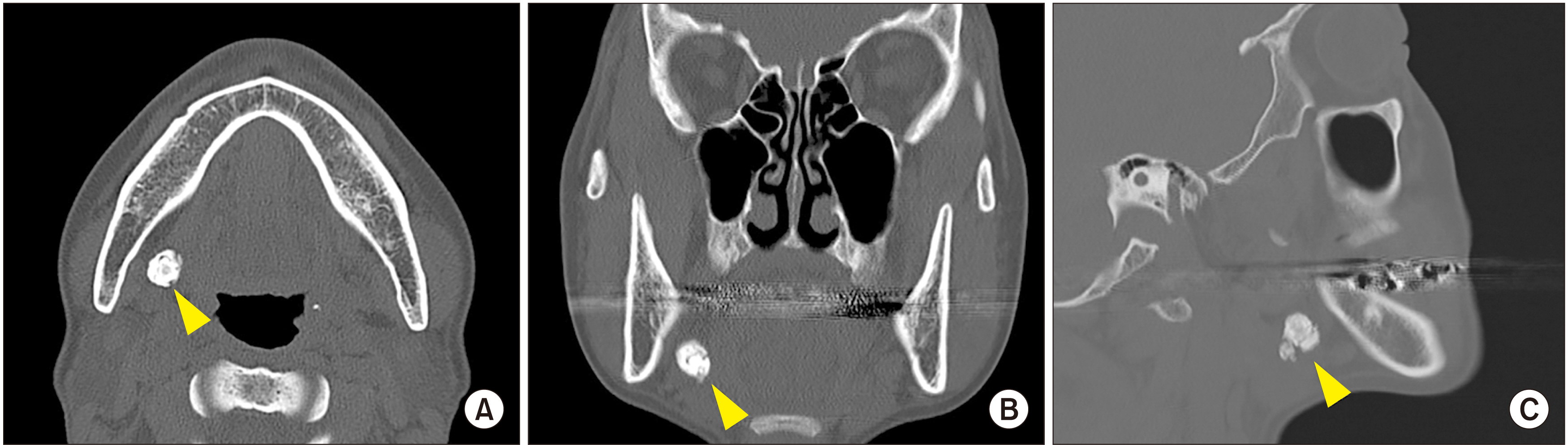
- The exact mechanism of sialolith formation has yet to be determined. Recurrence of sialolithiasis is rare, affecting only 1%-10% of patients. The current study presents a case of recurrent stones that occurred twice on the right submandibular gland 6 months postoperative and 7 months after reoperation in a 48-year-old female patient. The stones were analyzed using histology, scanning electron microscopy, energy dispersive spectroscopy, and transmission electron microscopy (TEM). The first stone showed a three-layered structure with a poorly mineralized peripheral multilayered zone, highly mineralized middle layer, and the central nidus. The stones were composed of Ca, C, O, Cu, F, N, P, Si, Zn, and Zr. In TEM, compact bi-layered bacterial cell membrane was found on the peripheral layer and the central nidus of the stone as well as exosomes in the central nidus. The results demonstrated the essential components of sialolith formation, including bacteria, inflammatory exosomes, and exfoliated salivary epithelial cells that cooperatively underwent the pathogenetic progresses of central nidus formation, induction of compact zone calcification of the middle layer, and repeated subsequent deposition in the peripheral multilayer zone. The rapid recurrence could have resulted from residual pieces of a sialolith acting as the nidus of bacterial infection.
Keyword
Figure
Reference
-
References
1. Barry R, Schaitkin BM, Walvekar RR. Gillespie MB, Walvekar RR, Schaitkin BM, Eisele DW, editors. 2018. Submandibular stones. Gland-preserving salivary surgery: a problem-based approach. Springer;p. 57–68. DOI: 10.1007/978-3-319-58335-8_6. PMCID: PMC6702475.
Article2. Lim HK, Kim SM, Kim MJ, Lee JH. 2012; Clinical, statistical and chemical study of sialolithiasis. J Korean Assoc Oral Maxillofac Surg. 38:44–9. https://doi.org/10.5125/jkaoms.2012.38.1.44. DOI: 10.5125/jkaoms.2012.38.1.44.
Article3. Koch M, Schapher M, Mantsopoulos K, Goncalves M, Iro H. 2019; Intraductal pneumatic lithotripsy after extended transoral duct surgery in submandibular sialolithiasis. Otolaryngol Head Neck Surg. 160:63–9. https://doi.org/10.1177/0194599818802224. DOI: 10.1177/0194599818802224. PMID: 30296893.
Article4. Nolasco P, Anjos AJ, Marques JM, Cabrita F, da Costa EC, Maurício A, et al. 2013; Structure and growth of sialoliths: computed microtomography and electron microscopy investigation of 30 specimens. Microsc Microanal. 19:1190–203. https://doi.org/10.1017/s1431927613001694. DOI: 10.1017/S1431927613001694. PMID: 24001782.
Article5. Austin T, Davis J, Chan T. 2004; Sialolithiasis of submandibular gland. J Emerg Med. 26:221–3. https://doi.org/10.1016/j.jemermed.2003.07.007. DOI: 10.1016/j.jemermed.2003.07.007. PMID: 14980352.
Article6. Schapher M, Koch M, Weidner D, Scholz M, Wirtz S, Mahajan A, et al. 2020; Neutrophil extracellular traps promote the development and growth of human salivary stones. Cells. 9:2139. https://doi.org/10.3390/cells9092139. DOI: 10.3390/cells9092139. PMID: 32971767. PMCID: PMC7564068.
Article7. Trujillo O, Drusin MA, Rahmati R. 2017; Rapid recurrent sialolithiasis: altered stone composition and potential factors for recurrence. Laryngoscope. 127:1365–8. https://doi.org/10.1002/lary.26357. DOI: 10.1002/lary.26357. PMID: 27753112.
Article8. Kraaij S, Karagozoglu KH, Forouzanfar T, Veerman EC, Brand HS. 2014; Salivary stones: symptoms, aetiology, biochemical composition and treatment. Br Dent J. 217:E23. https://doi.org/10.1038/sj.bdj.2014.1054. DOI: 10.1038/sj.bdj.2014.1054. PMID: 25476659.
Article9. Duong LT, Kakiche T, Ferré F, Nawrocki L, Bouattour A. 2019; Management of anterior submandibular sialolithiasis. J Oral Med Oral Surg. 25:16. https://doi.org/10.1051/mbcb/2018039. DOI: 10.1051/mbcb/2018039.
Article10. Galli P, Ceva A, Foletti JM, Iline N, Giorgi R, Chossegros C, et al. 2021; Salivary gland lithiasis recurrence after minimally-invasive surgery: incidence, risk factors and prevention. Laryngoscope. 131:794–9. https://doi.org/10.1002/lary.28991. DOI: 10.1002/lary.28991. PMID: 32786079.
Article11. Kim JK, Shin SM, Lee H, Lee S. 2016; Factors affecting long-term outcome of transoral surgery for submandibular stones: a follow-up study of 125 patients. Clin Otolaryngol. 41:365–70. https://doi.org/10.1111/coa.12523. DOI: 10.1111/coa.12523. PMID: 26292653.
Article12. Avishai G, Ben-Zvi Y, Ghanaiem O, Chaushu G, Gilat H. 2020; Sialolithiasis-do early diagnosis and removal minimize post-operative morbidity? Medicina (Kaunas). 56:332. https://doi.org/10.3390/medicina56070332. DOI: 10.3390/medicina56070332. PMID: 32630773. PMCID: PMC7404452.
Article13. Koch M, Mantsopoulos K, Müller S, Sievert M, Iro H. 2021; Treatment of sialolithiasis: what has changed? An update of the treatment algorithms and a review of the literature. J Clin Med. 11:231. https://doi.org/10.3390/jcm11010231. DOI: 10.3390/jcm11010231. PMID: 35011971. PMCID: PMC8746135.
Article14. Gerni M, Foletti JM, Collet C, Chossegros C. 2017; Evaluation of the prevalence of residual sialolith fragments after transoral approach of Wharton's duct. J Craniomaxillofac Surg. 45:167–70. https://doi.org/10.1016/j.jcms.2016.04.011. DOI: 10.1016/j.jcms.2016.04.011. PMID: 28040303.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Giant sialolithiasis of the submandibular gland: a case report
- A Case of Multiple Sialoliths in Sublingual Gland Misdiagnosed as Sialoliths in Submandibular Gland
- Histopathology and ultrastructural findings of pediatric sialolithiasis: a brief communication
- Sialolithiasis in children: Three case reports
- Submandibular sialolithiasis with CT and scintigraphy: CT values and salivary gland excretion in the submandibular glands