Acute Crit Care.
2024 Feb;39(1):34-46. 10.4266/acc.2023.00829.
The impact of ketamine on outcomes in critically ill patients: a systematic review with meta-analysis and trial sequential analysis of randomized controlled trials
- Affiliations
-
- 1Department of Mechanical and Aerospace Engineering, School of Engineering and Digital Sciences, Nazarbayev University, Astana, Kazakhstan
- 2Department of Emergency Medicine, Medical University of Vienna, Vienna, Austria
- 3Department of Surgery, School of Medicine, Nazarbayev University, Astana, Kazakhstan
- 4Department of Anesthesiology, Intensive Care, and Pain Medicine, National Research Oncology Center, Astana, Kazakhstan
- KMID: 2555222
- DOI: http://doi.org/10.4266/acc.2023.00829
Abstract
- Background
This meta-analysis aims to evaluate the effects of ketamine in critically ill intensive care unit (ICU) patients.
Methods
We searched for randomized controlled trials (RCTs) in PubMed, Scopus, and the Cochrane Library; the search was performed initially in January but was repeated in December of 2023. We focused on ICU patients of any age. We included studies that compared ketamine with other traditional agents used in the ICU. We synthesized evidence using RevMan v5.4 and presented the results as forest plots. We also used trial sequential analysis (TSA) software v. 0.9.5.10 Beta and presented results as TSA plots. For synthesizing results, we used a random-effects model and reported differences in outcomes of two groups in terms of mean difference (MD), standardized MD, and risk ratio with 95% confidence interval. We assessed the risk of bias using the Cochrane RoB tool for RCTs. Our outcomes were mortality, pain, opioid and midazolam requirements, delirium rates, and ICU length of stay.
Results
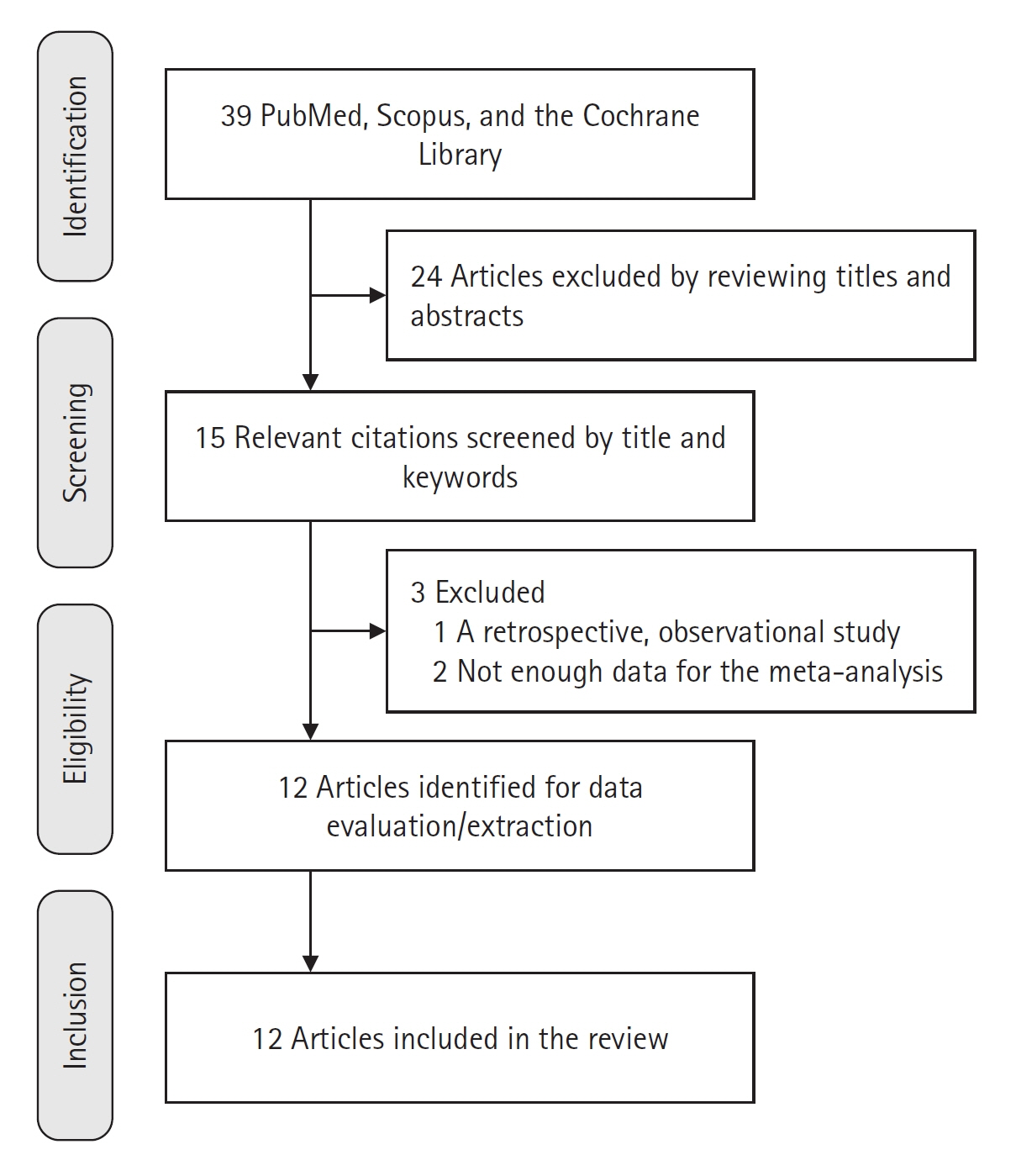
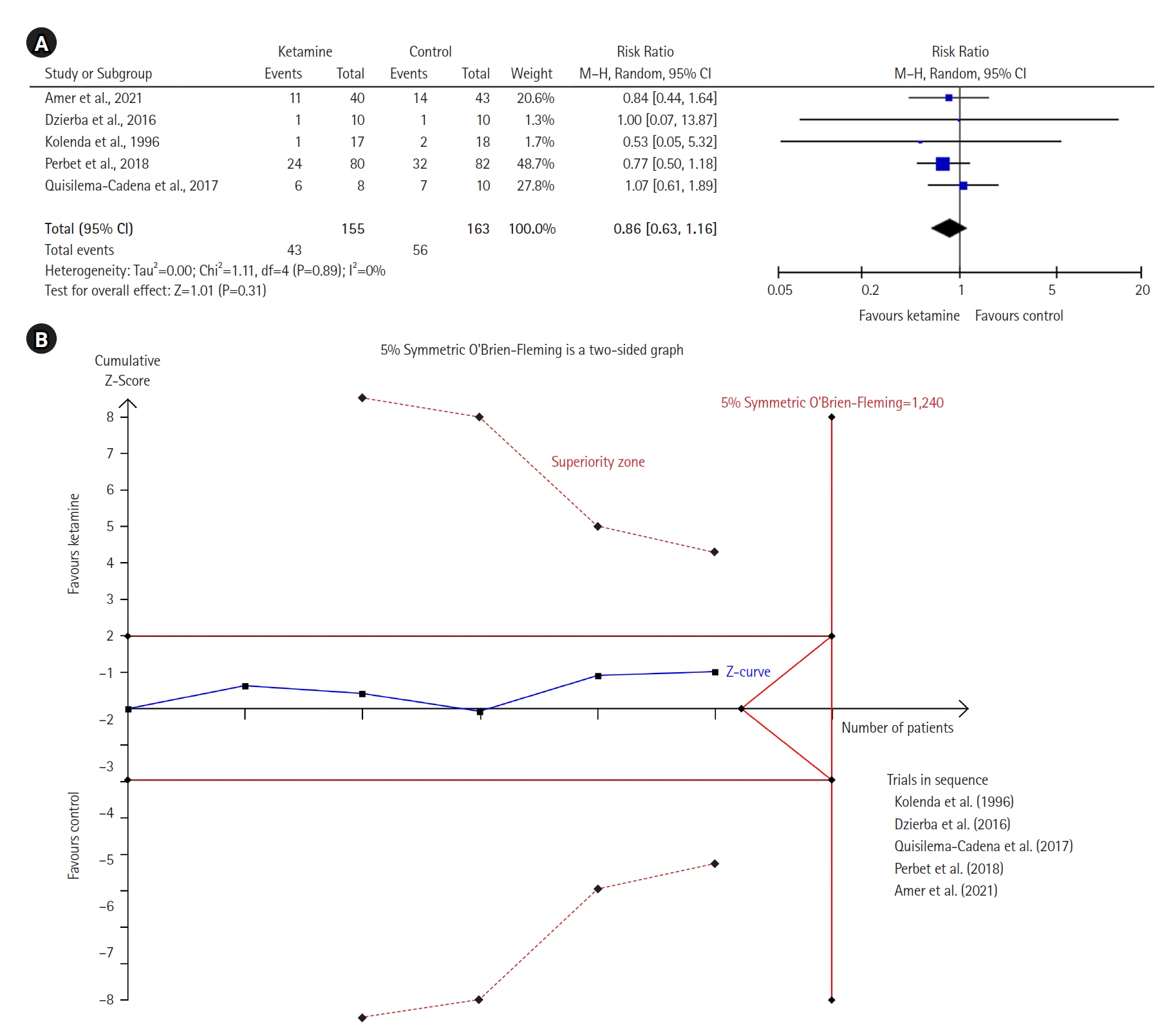
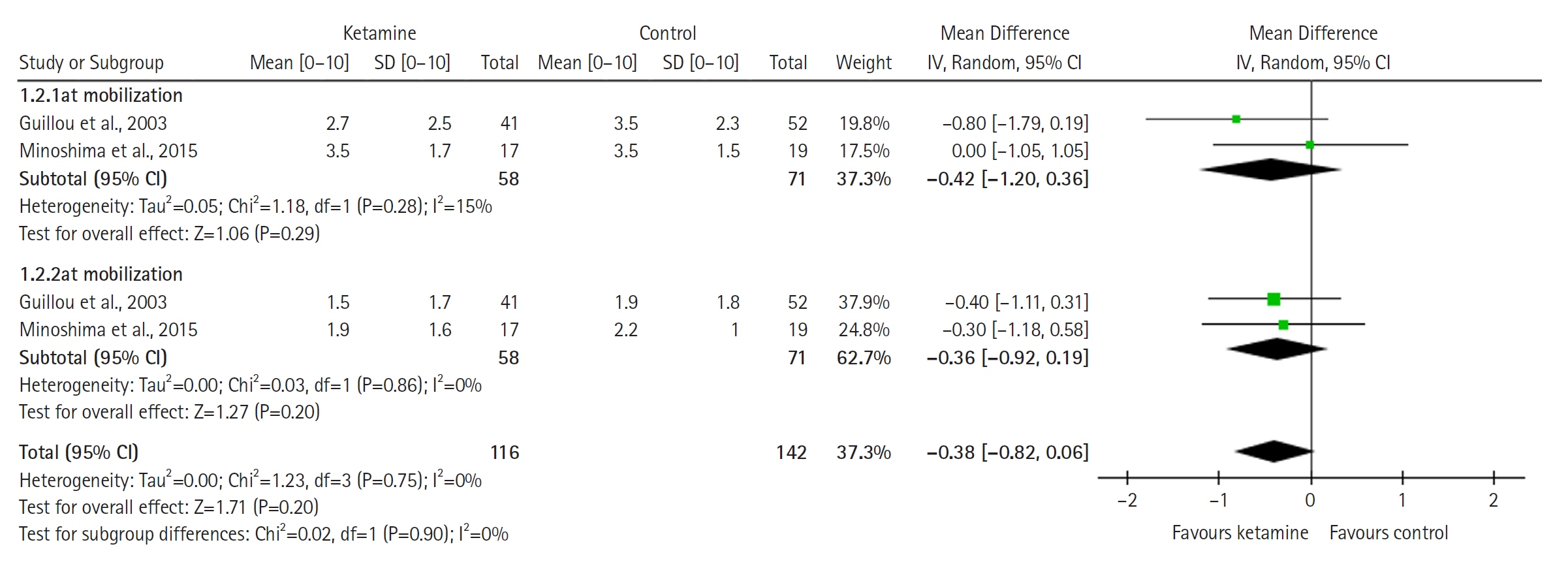
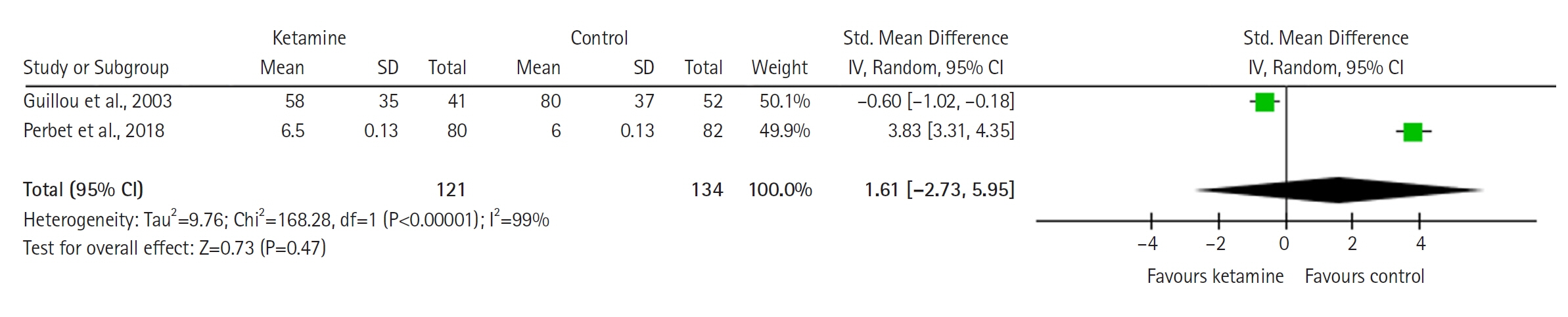
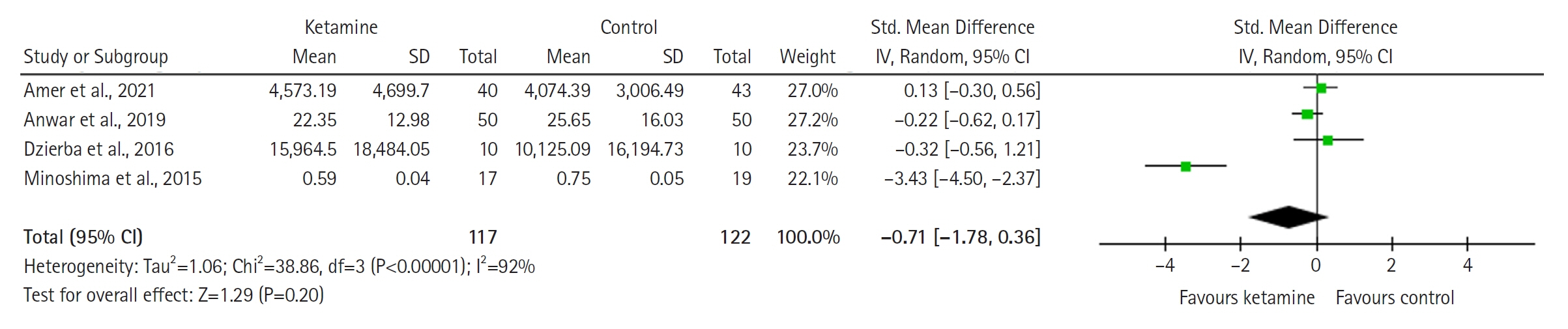
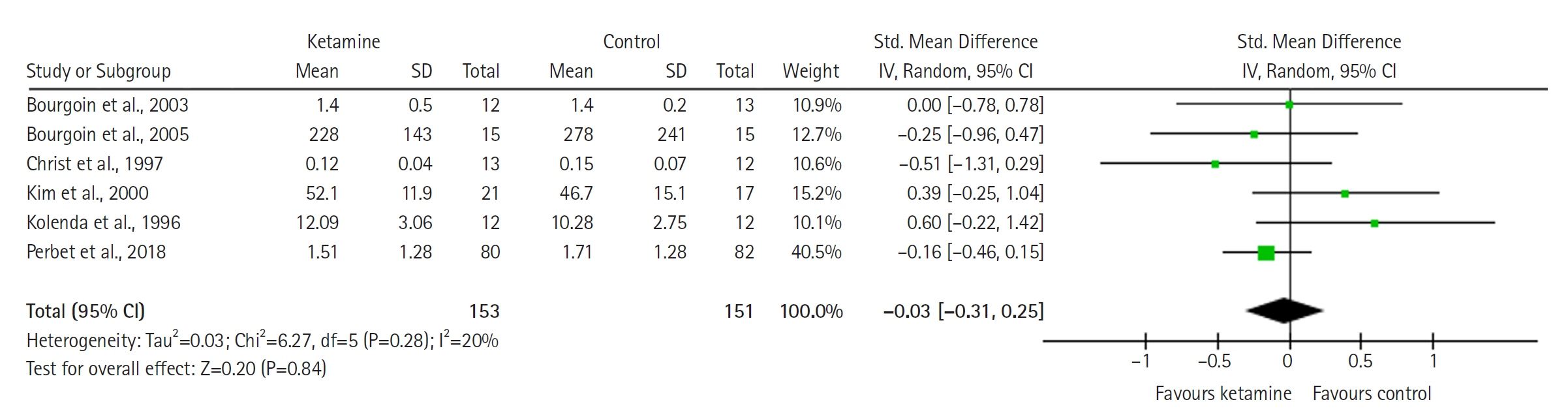
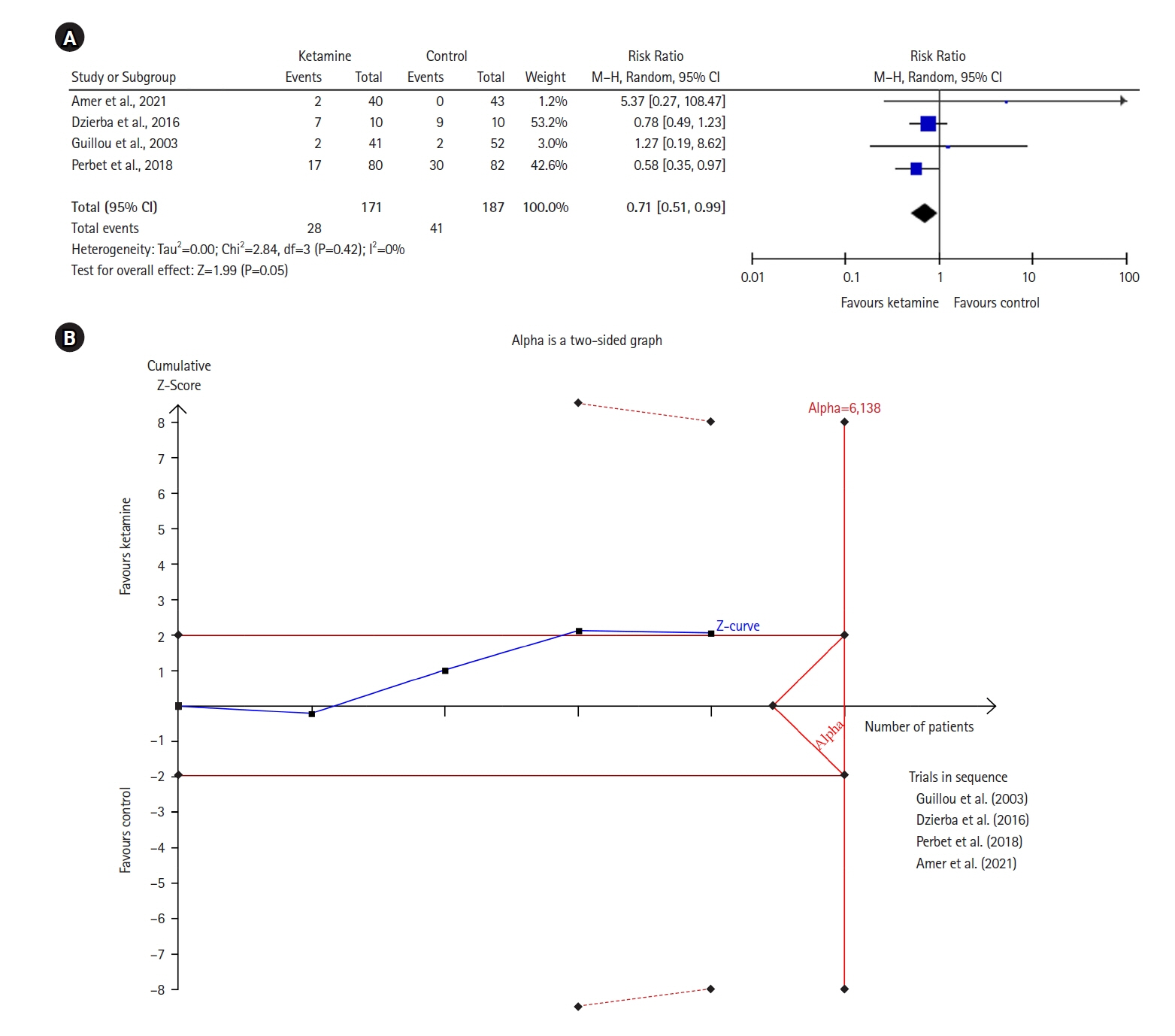
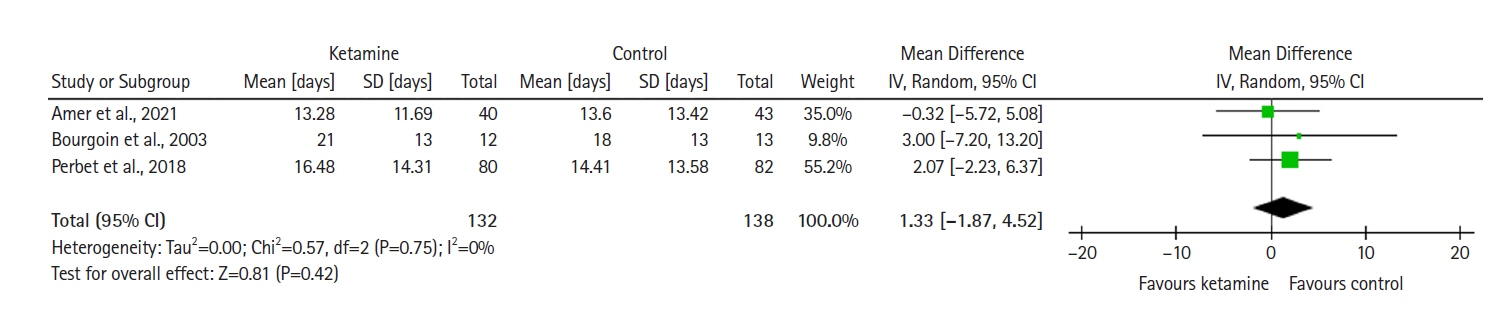
Twelve RCTs involving 805 ICU patients (ketamine group, n=398; control group, n=407) were included in the meta-analysis. The ketamine group was not superior to the control group in terms of mortality (in five studies with 318 patients), pain (two studies with 129 patients), mean and cumulative opioid consumption (six studies with 494 patients), midazolam consumption (six studies with 304 patients), and ICU length of stay (three studies with 270 patients). However, the model favored the ketamine group over the control group in delirium rate (four studies with 358 patients). This result is significant in terms of conventional boundaries (alpha=5%) but is not robust in sequential analysis. The applicability of the findings is limited by the small number of patients pooled for each outcome.
Conclusions
Our meta-analysis did not demonstrate differences between ketamine and control groups regarding any outcome except delirium rate, where the model favored the ketamine group over the control group. However, this result is not robust as sensitivity analysis and trial sequential analysis suggest that more RCTs should be conducted in the future.
Figure
Reference
-
1. Wheeler KE, Grilli R, Centofanti JE, Martin J, Gelinas C, Szumita PM, et al. Adjuvant analgesic use in the critically ill: a systematic review and meta-analysis. Crit Care Explor. 2020; 2:e0157.
Article2. Brinck EC, Tiippana E, Heesen M, Bell RF, Straube S, Moore RA, et al. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst Rev. 2018; 12:CD012033.
Article3. Chan K, Burry LD, Tse C, Wunsch H, De Castro C, Williamson DR. Impact of ketamine on analgosedative consumption in critically ill patients: a systematic review and meta-analysis. Ann Pharmacother. 2022; 56:1139–58.
Article4. Hurth KP, Jaworski A, Thomas KB, Kirsch WB, Rudoni MA, Wohlfarth KM. The reemergence of ketamine for treatment in critically ill adults. Crit Care Med. 2020; 48:899–911.
Article5. Wang H, Wang C, Wang Y, Tong H, Feng Y, Li M, et al. Sedative drugs used for mechanically ventilated patients in intensive care units: a systematic review and network meta-analysis. Curr Med Res Opin. 2019; 35:435–46.
Article6. Koo BN, Koh SO, Park SY, Shim JK, Chon SS. Continuous infusion of ketamine in mechanically ventilated patient in septic shock with status asthmaticus. Korean J Crit Care Med. 2000; 15:108–12.7. Kim TH, Lim CM, Shim TS, Lee SD, Kim WS, Kim DS, et al. Comparison of the efficacy between ketamine and morphine on sedation and analgesia in patients with mechanical ventilation. Korean J Crit Care Med. 2000; 15:82–7.8. Jabre P, Combes X, Lapostolle F, Dhaouadi M, Ricard-Hibon A, Vivien B, et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet. 2009; 374:293–300.
Article9. Hughes S. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. BET 3: is ketamine a viable induction agent for the trauma patient with potential brain injury. Emerg Med J. 2011; 28:1076–7.10. Knack SK, Prekker ME, Moore JC, Klein LR, Atkins AH, Miner JR, et al. The effect of ketamine versus etomidate for rapid sequence intubation on maximum sequential organ failure assessment score: a randomized clinical trial. J Emerg Med. 2023; 65:e371–82.
Article11. Holland D, Glober N, Christopher S, Zahn E, Lardaro T, O'Donnell D. Prehospital sedation with ketamine vs. midazolam: repeat sedation, intubation, and hospital outcomes. Am J Emerg Med. 2020; 38:1748–53.
Article12. Panzer O, Moitra V, Sladen RN. Pharmacology of sedative-analgesic agents: dexmedetomidine, remifentanil, ketamine, volatile anesthetics, and the role of peripheral Mu antagonists. Anesthesiol Clin. 2011; 29:587–605.
Article13. Buchheit JL, Yeh DD, Eikermann M, Lin H. Impact of low-dose ketamine on the usage of continuous opioid infusion for the treatment of pain in adult mechanically ventilated patients in surgical intensive care units. J Intensive Care Med. 2019; 34:646–51.
Article14. Hudetz JA, Patterson KM, Iqbal Z, Gandhi SD, Byrne AJ, Hudetz AG, et al. Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2009; 23:651–7.
Article15. Smischney NJ, Beach ML, Loftus RW, Dodds TM, Koff MD. Ketamine/propofol admixture (ketofol) is associated with improved hemodynamics as an induction agent: a randomized, controlled trial. J Trauma Acute Care Surg. 2012; 73:94–101.
Article16. Zeiler FA, Teitelbaum J, West M, Gillman LM. The ketamine effect on ICP in traumatic brain injury. Neurocrit Care. 2014; 21:163–73.
Article17. Hudetz JA, Pagel PS. Neuroprotection by ketamine: a review of the experimental and clinical evidence. J Cardiothorac Vasc Anesth. 2010; 24:131–42.
Article18. Chang LC, Raty SR, Ortiz J, Bailard NS, Mathew SJ. The emerging use of ketamine for anesthesia and sedation in traumatic brain injuries. CNS Neurosci Ther. 2013; 19:390–5.
Article19. Kumar A, Kohli A. Comeback of ketamine: resurfacing facts and dispelling myths. Korean J Anesthesiol. 2021; 74:103–14.
Article20. Von der Brelie C, Seifert M, Rot S, Tittel A, Sanft C, Meier U, et al. Sedation of patients with acute aneurysmal subarachnoid hemorrhage with ketamine is safe and might influence the occurrence of cerebral infarctions associated with delayed cerebral ischemia. World Neurosurg. 2017; 97:374–82.
Article21. Erstad BL, Patanwala AE. Ketamine for analgosedation in critically ill patients. J Crit Care. 2016; 35:145–9.
Article22. Manasco AT, Stephens RJ, Yaeger LH, Roberts BW, Fuller BM. Ketamine sedation in mechanically ventilated patients: a systematic review and meta-analysis. J Crit Care. 2020; 56:80–8.
Article23. Perbet S, Verdonk F, Godet T, Jabaudon M, Chartier C, Cayot S, et al. Low doses of ketamine reduce delirium but not opiate consumption in mechanically ventilated and sedated ICU patients: a randomised double-blind control trial. Anaesth Crit Care Pain Med. 2018; 37:589–95.
Article24. Hovaguimian F, Tschopp C, Beck-Schimmer B, Puhan M. Intraoperative ketamine administration to prevent delirium or postoperative cognitive dysfunction: a systematic review and meta-analysis. Acta Anaesthesiol Scand. 2018; 62:1182–93.
Article25. Mion G. Ketamine infusions for sedation in ICU. Anaesth Crit Care Pain Med. 2019; 38:397–8.
Article26. Perbet S, Godet T, Constantin JM. Ketamine infusion for sedation in ICU, response to Dr Mion. Anaesth Crit Care Pain Med. 2019; 38:399.
Article27. Viderman D, Aubakirova M, Nabidollayeva F, Yegembayeva N, Bilotta F, Badenes R, et al. Effect of ketamine on postoperative neurocognitive disorders: a systematic review and meta-analysis. J Clin Med. 2023; 12:4314.
Article28. Viderman D, Nabidollayeva F, Aubakirova M, Yessimova D, Badenes R, Abdildin Y. Postoperative delirium and cognitive dysfunction after general and regional anesthesia: a systematic review and meta-analysis. J Clin Med. 2023; 12:3549.
Article29. PRISMA [Internet]. PRISMA; 2023 [cited 2023 Apr 1]. Available from: http://www.prisma-statement.org/PRISMAStatement/.30. Thorlund K, Engstrøm J, Wetterslev J, Imberger G, Gluud C. Trial sequential analysis (TSA). Copenhagen Trial Unit; 2011.31. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018; 27:1785–805.
Article32. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014; 14:135.
Article33. Cochrane. RevMan [Internet]. Cochrane; 2023 [cited 2023 Apr 1]. Available from: https://training.cochrane.org/online-learning/core-software/revman.34. Kang H. Trial sequential analysis: novel approach for meta-analysis. Anesth Pain Med (Seoul). 2021; 16:138–50.
Article35. Sanfilippo F, La Via L, Tigano S, Morgana A, La Rosa V, Astuto M. Trial sequential analysis: the evaluation of the robustness of meta-analyses findings and the need for further research. EuroMediterranean Biomed J. 2021; 16:104–7.36. Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008; 61:64–75.
Article37. De Cassai A, Pasin L, Boscolo A, Salvagno M, Navalesi P. Trial sequential analysis: plain and simple. Korean J Anesthesiol. 2021; 74:363–5.
Article38. Castellini G, Bruschettini M, Gianola S, Gluud C, Moja L. Assessing imprecision in Cochrane systematic reviews: a comparison of GRADE and Trial Sequential Analysis. Syst Rev. 2018; 7:110.
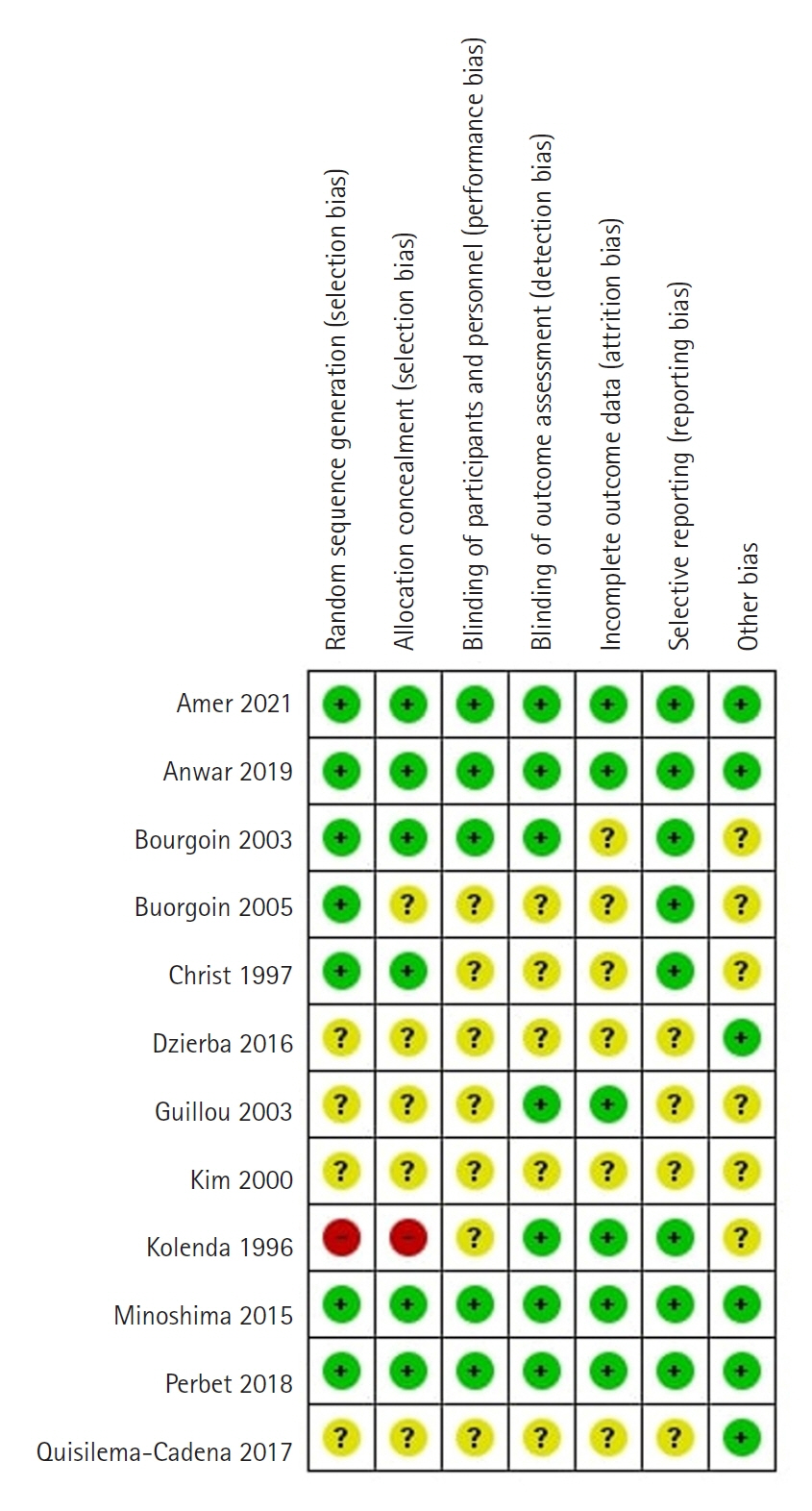
Article39. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.
Article40. Amer M, Maghrabi K, Bawazeer M, Alshaikh K, Shaban M, Rizwan M, et al. Adjunctive ketamine for sedation in critically ill mechanically ventilated patients: an active-controlled, pilot, feasibility clinical trial. J Intensive Care. 2021; 9:54.
Article41. Anwar S, Cooper J, Rahman J, Sharma C, Langford R. Prolonged perioperative use of pregabalin and ketamine to prevent persistent pain after cardiac surgery. Anesthesiology. 2019; 131:119–31.
Article42. Bawazeer M, Amer M, Maghrabi K, Alshaikh K, Amin R, Rizwan M, et al. Adjunct low-dose ketamine infusion vs standard of care in mechanically ventilated critically ill patients at a Tertiary Saudi Hospital (ATTAINMENT Trial): study protocol for a randomized, prospective, pilot, feasibility trial. Trials. 2020; 21:288.
Article43. Christ G, Mundigler G, Merhaut C, Zehetgruber M, Kratochwill C, Heinz G, et al. Adverse cardiovascular effects of ketamine infusion in patients with catecholamine-dependent heart failure. Anaesth Intensive Care. 1997; 25:255–9.
Article44. Garner O, Patterson J, Mejia JM, Anand V, Deleija J, Nemeh C, et al. Impact of ketamine as an adjunct sedative in acute respiratory distress syndrome due to COVID-19 Pneumonia. Respir Med. 2021; 189:106667.
Article45. Kolenda H, Gremmelt A, Rading S, Braun U, Markakis E. Ketamine for analgosedative therapy in intensive care treatment of head-injured patients. Acta Neurochir (Wien). 1996; 138:1193–9.
Article46. Quisilema-Cadena JM, Cordero-Escobar I, González-Hernández O. Comparación de dos esquemas de sedoanalgesia: Hermanos Ameijeiras. Revista Mexicana Anestesiología. 2017; 40:155–61.47. Schmittner MD, Vajkoczy SL, Horn P, Bertsch T, Quintel M, Vajkoczy P, et al. Effects of fentanyl and S(+)-ketamine on cerebral hemodynamics, gastrointestinal motility, and need of vasopressors in patients with intracranial pathologies: a pilot study. J Neurosurg Anesthesiol. 2007; 19:257–62.48. Guillou N, Tanguy M, Seguin P, Branger B, Campion JP, Mallédant Y. The effects of small-dose ketamine on morphine consumption in surgical intensive care unit patients after major abdominal surgery. Anesth Analg. 2003; 97:843–7.
Article49. Minoshima R, Kosugi S, Nishimura D, Ihara N, Seki H, Yamada T, et al. Intra- and postoperative low-dose ketamine for adolescent idiopathic scoliosis surgery: a randomized controlled trial. Acta Anaesthesiol Scand. 2015; 59:1260–8.
Article50. Dzierba AL, Brodie D, Bacchetta M, Muir J, Wasson L, Colabraro M, et al. Ketamine use in sedation management in patients receiving extracorporeal membrane oxygenation. Intensive Care Med. 2016; 42:1822–3.
Article51. Bourgoin A, Albanèse J, Wereszczynski N, Charbit M, Vialet R, Martin C. Safety of sedation with ketamine in severe head injury patients: comparison with sufentanil. Crit Care Med. 2003; 31:711–7.
Article52. Bourgoin A, Albanèse J, Léone M, Sampol-Manos E, Viviand X, Martin C. Effects of sufentanil or ketamine administered in target-controlled infusion on the cerebral hemodynamics of severely brain-injured patients. Crit Care Med. 2005; 33:1109–13.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Introduction to systematic review and meta-analysis
- The Impact of Mental Practice on Motor Function in Patients With Stroke: A Systematic Review and Meta-analysis
- Trial sequential analysis: novel approach for meta-analysis
- When and why to start continuous renal replacement therapy in critically ill patients with acute kidney injury
- Vitamin D and Risk of Respiratory Tract Infections in Children: A Systematic Review and Meta-analysis of Randomized Controlled Trials