Ann Clin Neurophysiol.
2024 Apr;26(1):14-21. 10.14253/acn.23013.
Significance of thalamic hyperperfusion patterns in computed tomography perfusion in patients with nonconvulsive status epilepticus: possible utility in predicting antiseizure medication resistance
- Affiliations
-
- 1Department of Neurology, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- KMID: 2554849
- DOI: http://doi.org/10.14253/acn.23013
Abstract
- Background
This study aimed to determine the characteristics of computed tomography perfusion (CTP) patterns and their utility in predicting antiseizure medication (ASM) resistance in patients with nonconvulsive status epilepticus (NCSE).
Methods
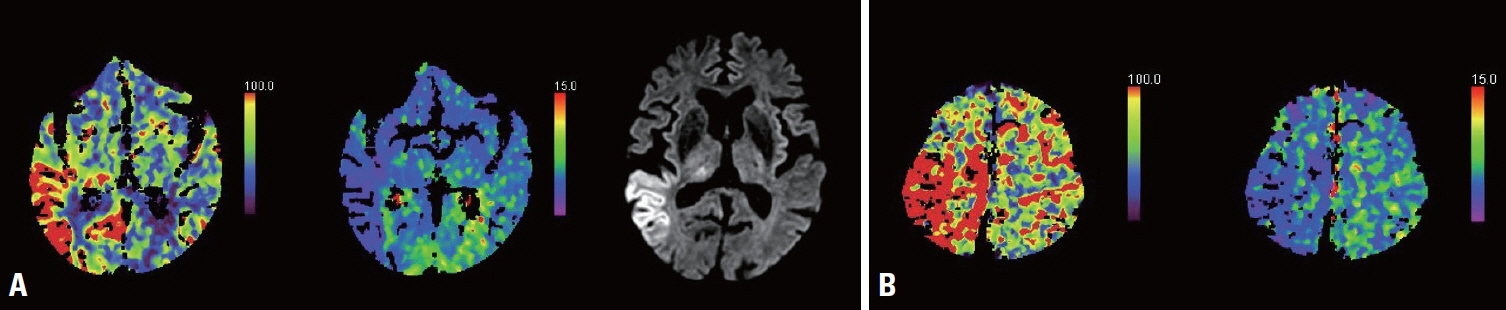
We retrospectively reviewed patients diagnosed with NCSE at Inje University Haeundae Paik Hospital Epilepsy Center between March 2015 and March 2022. CTP patterns were analyzed for those patients. A hyperperfusion pattern (HPP) in CTP was defined as a region of both increased cerebral blood flow and cerebral blood volume that did not necessarily follow the vascular territories. The primary endpoint was the responses to ASMs according to CTP patterns.
Results
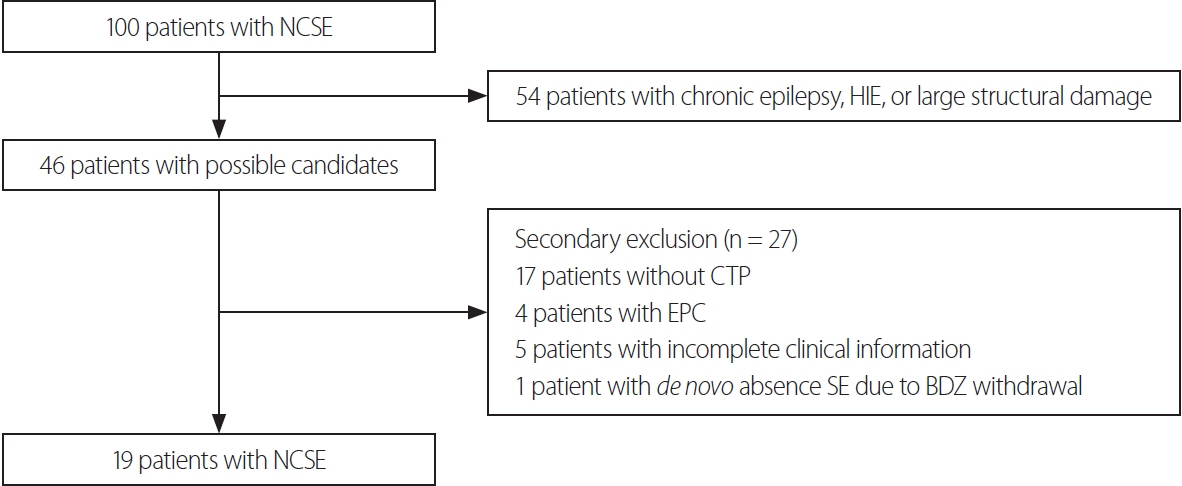
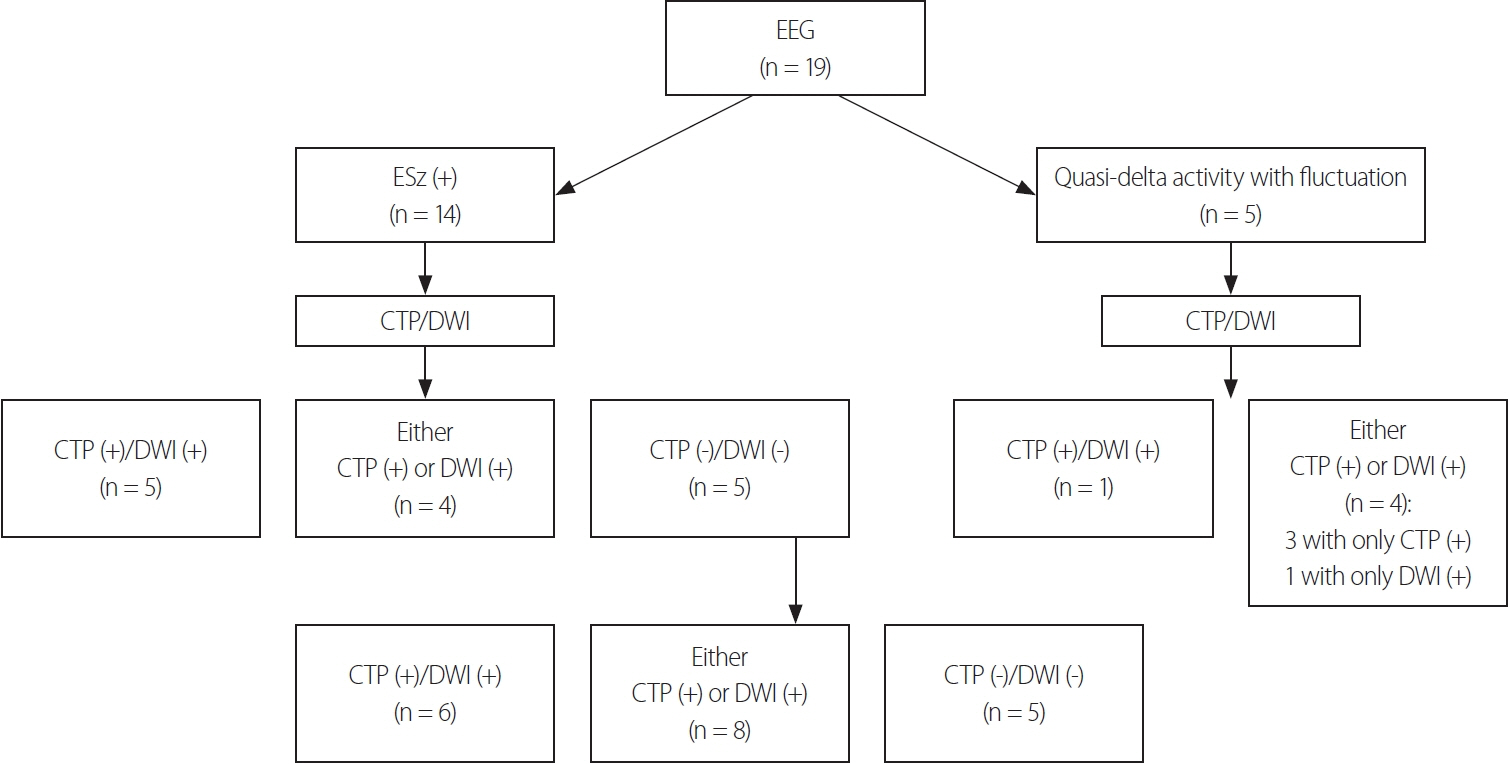
Fourteen (74%) of the 19 included patients met the criteria for definite NCSE, with the remaining 26% showing nonepileptiform activities with fluctuating quasirhythmic delta activities at >0.5 Hz on electroencephalogram. Three of the four patients who had HPPs with thalamic involvement were refractory to ASMs, whereas all eight patients who had HPPs without thalamic involvement were responsive to ASMs (p = 0.018). Although HPPs themselves were not associated with ASM responses, HPPs with thalamic involvement were associated with resistance to ASMs.
Conclusions
HPP with thalamic involvement in CTP might be associated with ASM resistance. Therefore, CTP may be useful for predicting ASM resistance in NCSE patients.
Keyword
Figure
Reference
-
1. Walker M, Cross H, Smith S, Young C, Aicardi J, Appleton R, et al. Nonconvulsive status epilepticus: epilepsy research foundation workshop reports. Epileptic Disord. 2005; 7:253–296.
Article2. Hauf M, Slotboom J, Nirkko A, von Bredow F, Ozdoba C, Wiest R. Cortical regional hyperperfusion in nonconvulsive status epilepticus measured by dynamic brain perfusion CT. AJNR Am J Neuroradiol. 2009; 30:693–698.
Article3. Sutter R, Kaplan PW. Electroencephalographic criteria for nonconvulsive status epilepticus: synopsis and comprehensive survey. Epilepsia. 2012; 53 Suppl 3:1–51.
Article4. Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996; 47:83–89.
Article5. Litt B, Wityk RJ, Hertz SH, Mullen PD, Weiss H, Ryan DD, et al. Nonconvulsive status epilepticus in the critically ill elderly. Epilepsia. 1998; 39:1194–1202.
Article6. Leitinger M, Beniczky S, Rohracher A, Gardella E, Kalss G, Qerama E, et al. Salzburg consensus criteria for non-convulsive status epilepticus--approach to clinical application. Epilepsy Behav. 2015; 49:158–163.
Article7. Leitinger M, Trinka E, Gardella E, Rohracher A, Kalss G, Qerama E, et al. Diagnostic accuracy of the Salzburg EEG criteria for non-convulsive status epilepticus: a retrospective study. Lancet Neurol. 2016; 15:1054–1062.
Article8. Goselink RJM, van Dillen JJ, Aerts M, Arends J, van Asch C, van der Linden I, et al. The difficulty of diagnosing NCSE in clinical practice; external validation of the Salzburg criteria. Epilepsia. 2019; 60:e88–e92.
Article9. Davies E, Elnagi F, Smith T. CT perfusion: stroke, seizure or both? BMJ Case Rep. 2021; 14:e245723.
Article10. Giovannini G, Malagoli M, Turchi G, Miani A, Orlandi N, Vaudano AE, et al. Cortical and thalamic hyper-perfusion in non-convulsive status epilepticus. Relationship between perfusion CT patterns and Salzburg EEG criteria. Seizure. 2021; 92:10–17.
Article11. Khoo CS, Kim SE, Lee BI, Shin KJ, Ha SY, Park J, et al. Characteristics of perfusion computed tomography imaging in patients with seizures mimicking acute stroke. Eur Neurol. 2020; 83:56–64.
Article12. Gugger JJ, Llinas RH, Kaplan PW. The role of CT perfusion in the evaluation of seizures, the post-ictal state, and status epilepticus. Epilepsy Res. 2020; 159:106256.
Article13. Sheikh IS, Park S, Ali I. Using CT perfusion in the interictal state. J Clin Neurophysiol. 2021; 38:e25–e28.
Article14. Leitinger M, Trinka E, Zimmermann G, Beniczky S. Salzburg criteria for nonconvulsive status epilepticus: details matter. Epilepsia. 2019; 60:2334–2336.
Article15. Gelfand JM, Wintermark M, Josephson SA. Cerebral perfusion-CT patterns following seizure. Eur J Neurol. 2010; 17:594–601.
Article16. Lucas L, Gariel F, Menegon P, Aupy J, Thomas B, Tourdias T, et al. Acute ischemic stroke or epileptic seizure? Yield of CT perfusion in a “code stroke” situation. AJNR Am J Neuroradiol. 2021; 42:49–56.
Article17. Williams J, Mullins G, Delanty N, Bede P, Doherty CP. The spectrum of peri-ictal MRI changes; four illustrative cases. Seizure. 2017; 50:189–193.
Article18. Samanta D, Garrity L, Arya R. Refractory and super-refractory status epilepticus. Indian Pediatr. 2020; 57:239–253.
Article19. Husain AM, Horn GJ, Jacobson MP. Non-convulsive status epilepticus: usefulness of clinical features in selecting patients for urgent EEG. J Neurol Neurosurg Psychiatry. 2003; 74:189–191.
Article20. Lee JJ, Park KI, Park JM, Kang K, Kwon O, Lee WW, et al. Clinical characteristics and treatment outcomes of de novo nonconvulsive status epilepticus: a retrospective study. J Clin Neurol. 2021; 17:26–32.
Article21. Gschwind M, Foletti G, Baumer A, Bottani A, Novy J. Recurrent nonconvulsive status epilepticus in a patient with coffin-lowry syndrome. Mol Syndromol. 2015; 6:91–95.
Article22. Stackhouse TL, Mishra A. Neurovascular coupling in development and disease: focus on astrocytes. Front Cell Dev Biol. 2021; 9:702832.
Article23. Phillips AA, Chan FH, Zheng MM, Krassioukov AV, Ainslie PN. Neurovascular coupling in humans: physiology, methodological advances and clinical implications. J Cereb Blood Flow Metab. 2016; 36:647–664.
Article24. Kaplan L, Chow BW, Gu C. Neuronal regulation of the bloodbrain barrier and neurovascular coupling. Nat Rev Neurosci. 2020; 21:416–432.
Article25. Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002; 43:219–227.
Article26. Rosenberg DS, Mauguière F, Catenoix H, Faillenot I, Magnin M. Reciprocal thalamocortical connectivity of the medial pulvinar: a depth stimulation and evoked potential study in human brain. Cereb Cortex. 2009; 19:1462–1473.
Article27. Stein T, Moritz C, Quigley M, Cordes D, Haughton V, Meyerand E. Functional connectivity in the thalamus and hippocampus studied with functional MR imaging. AJNR Am J Neuroradiol. 2000; 21:1397–1401.28. Lee DA, Lee J, Kim HC, Park KM, Kim SE. Hippocampal injury in patients with status epilepticus: quantitative analysis of hippocampal volume and structural co-variance network. Seizure. 2022; 95:84–89.
Article29. Navidhamidi M, Ghasemi M, Mehranfard N. Epilepsy-associated alterations in hippocampal excitability. Rev Neurosci. 2017; 28:307–334.
Article30. Fisher RS. Deep brain stimulation of thalamus for epilepsy. Neurobiol Dis. 2023; 179:106045.31. Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019; 393:689–701.
Article32. Kremkow J, Alonso JM. Thalamocortical circuits and functional architecture. Annu Rev Vis Sci. 2018; 4:263–285.
Article33. Rodriguez-Moreno J, Porrero C, Rollenhagen A, Rubio-Teves M, Casas-Torremocha D, Alonso-Nanclares L, et al. Area-specific synapse structure in branched posterior nucleus axons reveals a new level of complexity in thalamocortical networks. J Neurosci. 2020; 40:2663–2679.
Article34. Lux HD, Heinemann U, Dietzel I. Ionic changes and alterations in the size of the extracellular space during epileptic activity. Adv Neurol. 1986; 44:619–639.35. Jabeen SA, Cherukuri P, Mridula R, Harshavardhana KR, Gaddamanugu P, Sarva S, et al. A prospective study of diffusion weighted magnetic resonance imaging abnormalities in patients with cluster of seizures and status epilepticus. Clin Neurol Neurosurg. 2017; 155:70–74.36. Hochman DW. The extracellular space and epileptic activity in the adult brain: explaining the antiepileptic effects of furosemide and bumetanide. Epilepsia. 2012; 53 Suppl 1:18–25.
Article37. Mendes A, Sampaio L. Brain magnetic resonance in status epilepticus: a focused review. Seizure. 2016; 38:63–67.
Article38. Lee DA, Park KM, Kim HC, Khoo CS, Lee BI, Kim SE. Spectrum of ictal-interictal continuum: the significance of 2HELPS2B score and background suppression. J Clin Neurophysiol. 2023; 40:364–370.
Article39. Abend NS, Dlugos DJ, Hahn CD, Hirsch LJ, Herman ST. Use of EEG monitoring and management of non-convulsive seizures in critically ill patients: a survey of neurologists. Neurocrit Care. 2010; 12:382–389.
Article40. Towne AR, Waterhouse EJ, Boggs JG, Garnett LK, Brown AJ, Smith JR Jr, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000; 54:340–345.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 18F-FDG PET and 99mTc-ECD SPECT between Ictal and Interictal Phase in a Patient with Status Epilepticus Arising from the Occipital Lobe
- Nonconvulsive Status Epilepticus Associated with Hashimoto's Encephalopathy
- A Case of Complex Partial Status Epilepticus
- Contrast-induced encephalopathy and nonconvulsive status epilepticus after diagnostic cerebral angiography in an end-stage renal disease patient
- Ceftazidime-Induced Nonconvulsive Status Epilepticus