Ann Pediatr Endocrinol Metab.
2024 Apr;29(2):109-118. 10.6065/apem.2346046.023.
Long-term endocrine sequelae after hematopoietic stem cell transplantation in children and adolescents
- Affiliations
-
- 1Department of Pediatrics, Asan Medical Center Children's Hospital, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Pediatrics, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea
- 3Department of Pediatrics, CHA Bundang Medical Center, CHA University, Seongnam, Korea
- KMID: 2554682
- DOI: http://doi.org/10.6065/apem.2346046.023
Abstract
- Purpose
As the survival rate from pediatric cancers has increased significantly with advances in treatment modalities, long-term endocrine complications have also risen. This study investigated the frequencies and risks of endocrine sequelae in childhood cancer survivors who received hematopoietic stem cell transplantation (HSCT).
Methods
This study included 200 pediatric patients who underwent HSCT. Clinical and endocrinological findings were collected retrospectively. The median follow-up duration after HSCT was 14 years.
Results
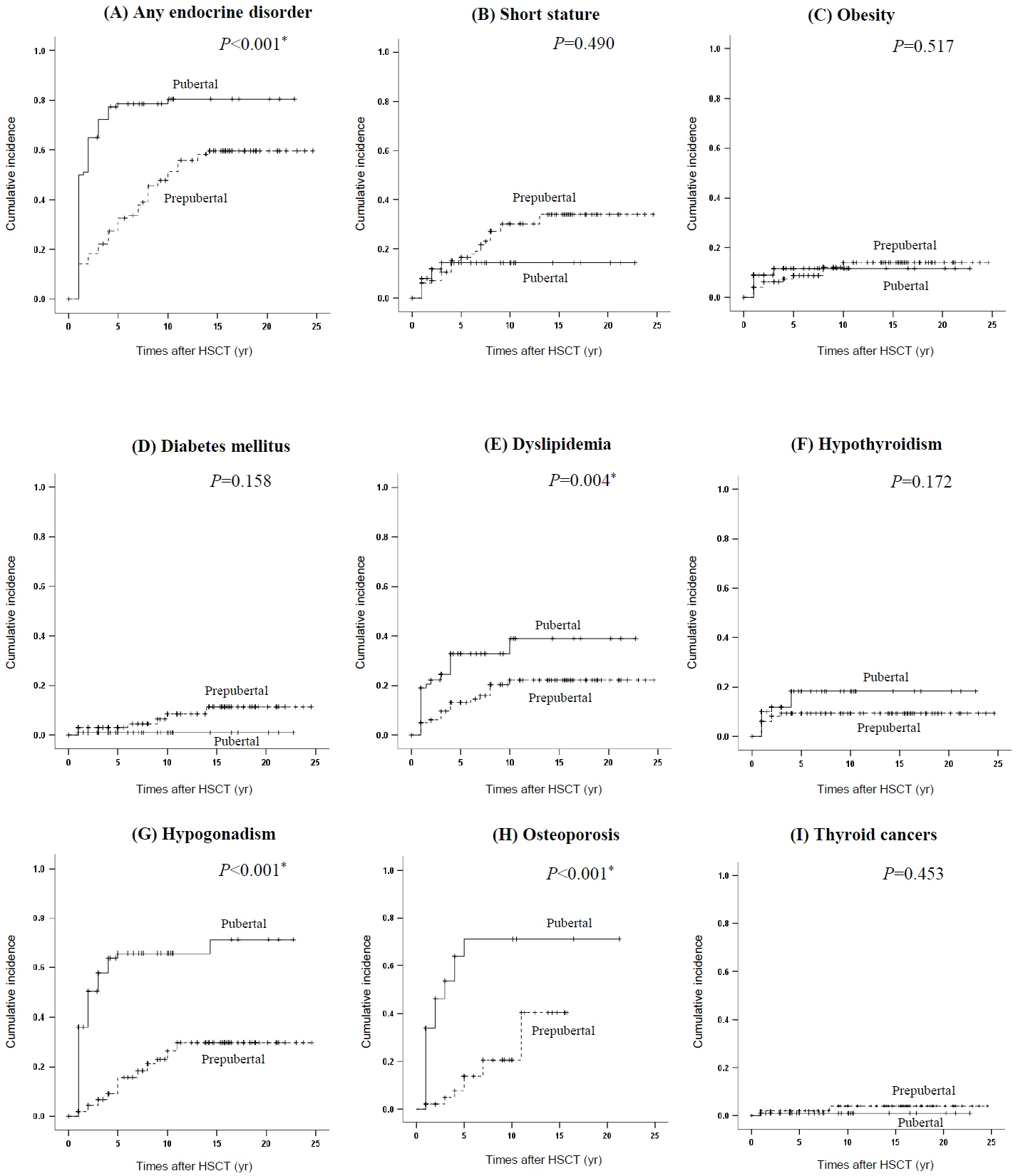
Endocrine complications occurred in 135 patients (67.5%). Children who underwent HSCT at pubertal age (n=100) were at higher risk of endocrine complications than those who received it at prepubertal age (79% vs. 56%, P=0.001). The most common complication was hypogonadism (40%), followed by dyslipidemia (22%). Short stature and diabetes mellitus were more prevalent in the prepubertal group, whereas hypogonadism and osteoporosis were more common in the pubertal group. Being female, pubertal age at HSCT, and glucocorticoid use were predictors of an increased risk for any complication. Radiation exposure increased the risk of short stature and hypothyroidism. Hypogonadism was significantly associated with being female, pubertal age at HSCT, and high-dose radiation. Pubertal age at HSCT also increased the risks of osteoporosis and dyslipidemia.
Conclusion
This study demonstrates that long-term endocrine complications are common after HSCT in children and adolescents. Age at HSCT is a critical factor for endocrine complications after HSCT. These findings suggest that surveillance strategies for endocrine complications in childhood cancer survivors should be specified according to age at HSCT.
Figure
Reference
-
References
1. Snowden JA, Sanchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant. 2022; 57:1217–39.
Article2. Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006; 355:1572–82.
Article3. Annaloro C, Usardi P, Airaghi L, Giunta V, Forti S, Orsatti A, et al. Prevalence of metabolic syndrome in long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008; 41:797–804.
Article4. Wei C, Albanese A. Endocrine disorders in childhood cancer survivors treated with haemopoietic stem cell transplantation. Children (Basel). 2014; 1:48–62.
Article5. Gebauer J, Higham C, Langer T, Denzer C, Brabant G. Long-term endocrine and metabolic consequences of cancer treatment: a systematic review. Endocr Rev. 2019; 40:711–67.
Article6. Akirov A, Sawka AM, Ben-Barouch S, Lipton J, Ezzat S. Endocrine complications in patients with GVHD. Endocr Pract. 2019; 25:485–90.
Article7. Brignardello E, Felicetti F, Castiglione A, Chiabotto P, Corrias A, Fagioli F, et al. Endocrine health conditions in adult survivors of childhood cancer: the need for specialized adult-focused follow-up clinics. Eur J Endocrinol. 2013; 168:465–72.
Article8. Shalitin S, Pertman L, Yackobovitch-Gavan M, Yaniv I, Lebenthal Y, Phillip M, et al. Endocrine and metabolic disturbances in survivors of hematopoietic stem cell transplantation in childhood and adolescence. Horm Res Paediatr. 2018; 89:108–21.
Article9. Gokcebay DG, Azik F, Bayram C, Erdem AY, Fettah A, Isik P, et al. Evaluation of endocrine and metabolic dysfunctions after hematopoietic stem cell transplantation in children: a study from Turkey. J Pediatr Endocrinol Metab. 2017; 30:683–91.
Article10. Sanders JE. Endocrine complications of high-dose therapy with stem cell transplantation. Pediatr Transplant. 2004; 8 Suppl 5:39–50.
Article11. Jung MH, Cho KS, Lee JW, Chung NG, Cho B, Suh BK, et al. Endocrine complications after hematopoietic stem cell transplantation during childhood and adolescence. J Korean Med Sci. 2009; 24:1071–7.
Article12. de Kloet LC, Bense JE, van der Stoep M, Louwerens M, von Asmuth EGJ, Lankester AC, et al. Late endocrine effects after hematopoietic stem cell transplantation in children with nonmalignant diseases. Bone Marrow Transplant. 2022; 57:1564–72.
Article13. Green DM, Nolan VG, Goodman PJ, Whitton JA, Srivastava D, Leisenring WM, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014; 61:53–67.
Article14. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015; 21:389–401. e1.
Article15. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018; 61:135–49.
Article16. Lim JS, Kim EY, Kim JH, Yoo JH, Yi KH, Chae HW, et al. 2017 Clinical practice guidelines for dyslipidemia of Korean children and adolescents. Ann Pediatr Endocrinol Metab. 2020; 25:199–207.
Article17. Committee for the Korean Guidelines for the Management of Dyslipidemia. 2015 Korean Guidelines for the Management of Dyslipidemia: Executive Summary (English Translation). Korean Circ J. 2016; 46:275–306.18. Viswanathan V, Eugster EA. Etiology and treatment of hypogonadism in adolescents. Pediatr Clin North Am. 2011; 58:1181–200, x.
Article19. Kang MJ, Hong HS, Chung SJ, Lee YA, Shin CH, Yang SW. Body composition and bone density reference data for Korean children, adolescents, and young adults according to age and sex: results of the 2009-2010 Korean National Health and Nutrition Examination Survey (KNHANES). J Bone Miner Metab. 2016; 34:429–39.
Article20. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96:1911–30.
Article21. Lee Y, Shin J, Choi Y, Kim H, Koh KN, Im HJ, et al. Endocrine complications in children and adolescents with non-central nervous system solid tumors. Front Endocrinol (Lausanne). 2021; 12:610730.
Article22. Nowak J. Role of HLA in hematopoietic SCT. Bone Marrow Transplant. 2008; 42 Suppl 2:S71–6.
Article23. Koh LP, Rizzieri DA, Chao NJ. Allogeneic hematopoietic stem cell transplant using mismatched/haploidentical donors. Biol Blood Marrow Transplant. 2007; 13:1249–67.
Article24. Leung W, Ahn H, Rose SR, Phipps S, Smith T, Gan K, et al. A prospective cohort study of late sequelae of pediatric allogeneic hematopoietic stem cell transplantation. Medicine (Baltimore). 2007; 86:215–24.
Article25. Baker KS, Ness KK, Weisdorf D, Francisco L, Sun CL, Forman S, et al. Late effects in survivors of acute leukemia treated with hematopoietic cell transplantation: a report from the B one Marrow Transplant Sur vivor Study. Leukemia. 2010; 24:2039–47.
Article26. Golds G, Houdek D, Arnason T. Male hypogonadism and osteoporosis: the effects, clinical consequences, and treatment of testosterone deficiency in bone health. Int J Endocrinol. 2017; 2017:4602129.
Article27. Ozbek MN, Demirbilek H, Baran RT, Baran A. Bone mineral density in adolescent girls with hypogonadotropic and hypergonadotropic hypogonadism. J Clin Res Pediatr Endocrinol. 2016; 8:163–9.
Article28. Bhatnagar R, Dixit NM, Yang EH, Sallam T. Cancer therapy's impact on lipid metabolism: Mechanisms and future avenues. Front Cardiovasc Med. 2022; 9:925816.
Article29. Blaes A, Konety S, Hurley P. Cardiovascular complications of hematopoietic stem cell transplantation. Curr Treat Options Cardiovasc Med. 2016; 18:25.
Article30. Griffith ML, Jagasia M, Jagasia SM. Diabetes mellitus after hematopoietic stem cell transplantation. Endocr Pract. 2010; 16:699–706.
Article31. Kim HJ, Kim YM, Kang E, Lee BH, Choi JH, Yoo HW. Diabetes mellitus caused by secondary hemochromatosis after multiple blood transfusions in 2 patients with severe aplastic anemia. Ann Pediatr Endocrinol Metab. 2017; 22:60–4.
Article32. Allen CM, Lopes F, Mitchell RT, Spears N. How does chemotherapy treatment damage the prepubertal testis? Reproduction. 2018; 156:R209–33.
Article33. Teinturier C, Hartmann O, Valteau-Couanet D, Benhamou E, Bougneres PF. Ovarian function after autologous bone marrow transplantation in childhood: high-dose busulfan is a major cause of ovarian failure. Bone Marrow Transplant. 1998; 22:989–94.
Article34. Howell S, Shalet S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol Metab Clin North Am. 1998; 27:927–43.
Article35. Rahal I, Galambrun C, Bertrand Y, Garnier N, Paillard C, Frange P, et al. Late effects after hematopoietic stem cell transplantation for beta-thalassemia major: the French national experience. Haematologica. 2018; 103:1143–9.
Article36. Paetow U, Bader P, Chemaitilly W. A systematic approach to the endocrine care of survivors of pediatric hematopoietic stem cell transplantation. Cancer Metastasis Rev. 2020; 39:69–78.
Article37. Hows JM, Passweg JR, Tichelli A, Locasciulli A, Szydlo R, Bacigalupo A, et al. Comparison of long-term outcomes after allogeneic hematopoietic stem cell transplantation from matched sibling and unrelated donors. Bone Marrow Transplant. 2006; 38:799–805.
Article38. Cohen A, Rovelli A, Bakker B, Uderzo C, van Lint MT, Esperou H, et al. Final height of patients who underwent bone marrow transplantation for hematological disorders during childhood: a study by the Working Party for Late Effects-EBMT. Blood. 1999; 93:4109–15.39. Bakker B, Oostdijk W, Geskus RB, Stokvis-Brantsma WH, Vossen JM, Wit JM. Patterns of growth and body proportions after total-body irradiation and hematopoietic stem cell transplantation during childhood. Pediatr Res. 2006; 59:259–64.
Article40. Wallace WH, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod. 2003; 18:117–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Commentary on "Long-term endocrine sequelae after hematopoietic stem cell transplantation in children and adolescents"
- Hematopoietic stem cell transplantation: overview for general pediatrician
- Opening the era of in vivo xenotransplantation model for hematopoietic stem cell transplantation
- Hematopoietic Stem Cell Transplantation
- Hematopoietic Stem Cell Transplantation in Inborn Error of Metabolism