Ann Pediatr Endocrinol Metab.
2024 Apr;29(2):82-89. 10.6065/apem.2346162.081.
Safety and tolerability of sodium-glucose cotransporter-2 inhibitors in children and young adults: a systematic review and meta-analysis
- Affiliations
-
- 1Department of Endocrinology, JSS Medical College, JSS Academy of Higher Education and Research, Mysore, India
- 2Department of Endocrinology, Center for Endocrinology Diabetes Arthritis & Rheumatism (CEDAR) Superspeciality Healthcare, Dwarka, New Delhi, India
- 3Department of Anaesthesiology, JSS Academy of Higher Education and Research, Mysore, India
- 4Department of Endocrinology, Khandelwal Diabetes, Thyroid & Endocrinology Clinic, Paschim Vihar, New Delhi, India
- 5Department of Endocrinology, Apollo Hospitals, Navi Mumbai, India
- 6Department of Rheumatology, Center for Endocrinology Diabetes Arthritis & Rheumatism (CEDAR) Superspeciality Healthcare, Dwarka, New Delhi, India
- KMID: 2554678
- DOI: http://doi.org/10.6065/apem.2346162.081
Abstract
- Purpose
Sodium glucose cotransporter-2 inhibitors (SGLT2i) have been evaluated in children with type 2 diabetes mellitus (T2DM), type 1 diabetes mellitus (T1DM), and several other nondiabetic conditions. Potential tolerability issues have prevented the routine use of SGLT2i in children with diabetes. However, no meta-analysis to date has evaluated the safety and tolerability of SGLT2i in children. This systematic review and meta-analysis aimed to address this knowledge gap.
Methods
Databases were searched for randomized controlled trials (RCTs), case control, and cohort studies involving children receiving SGLT2i in the intervention-arm. Primary outcome was occurrence of treatment emergent adverse events (TAEs). Secondary outcomes were evaluation of glycemic efficacy and occurrence of severe adverse events (SAEs), hypoglycemia, ketosis, genital or urinary infections, and any other adverse events.
Results
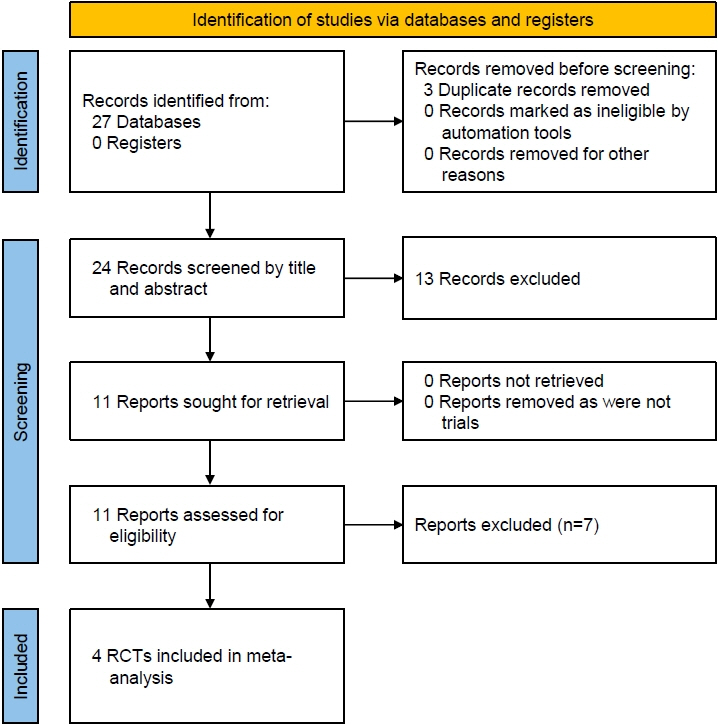
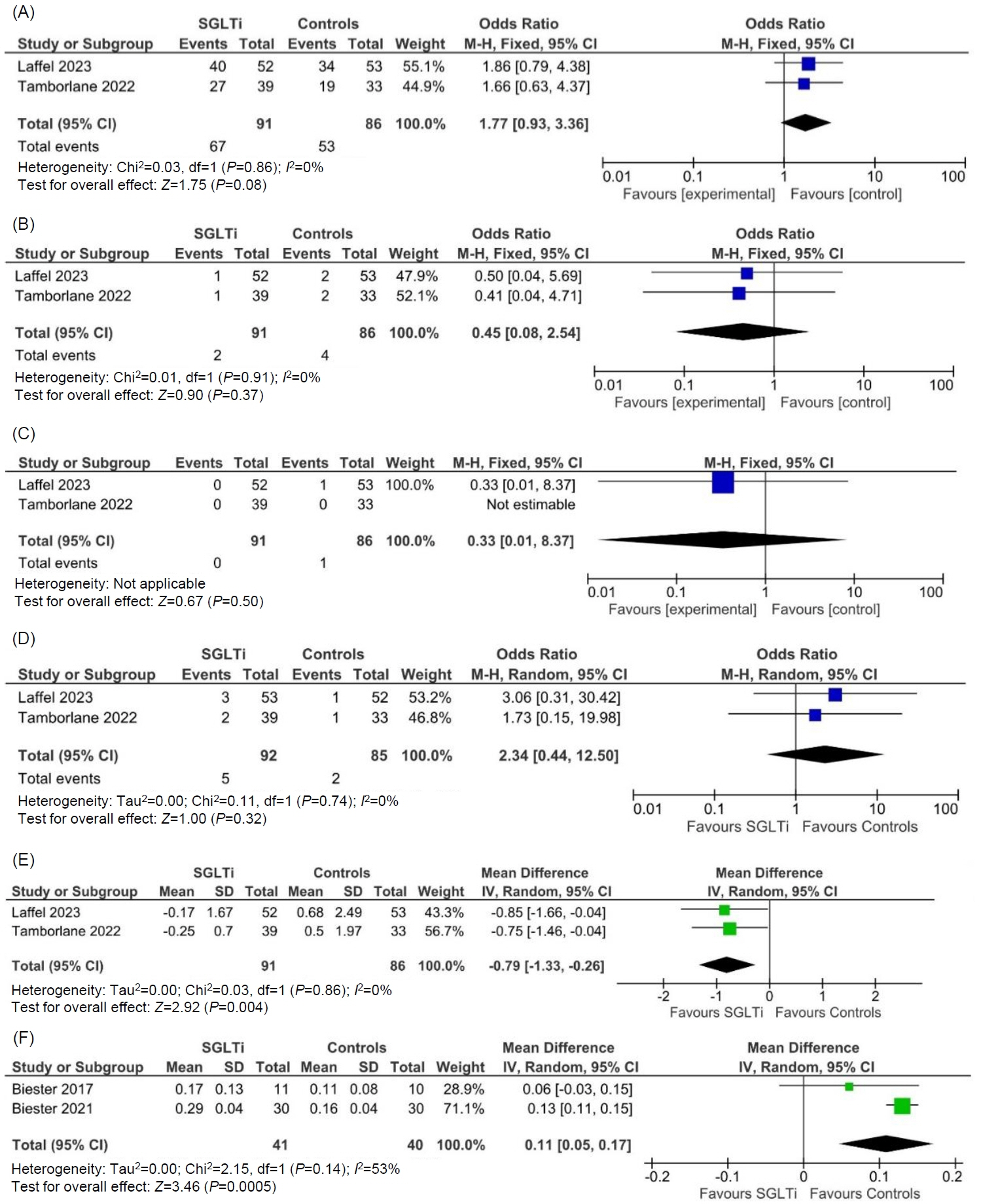
From the 27 articles initially screened, data from 4 RCTs (258 children) were analyzed. In children with T2DM, occurrence of TAEs (odds ratio [OR], 1.77; 95% confidence interval [CI], 0.93–3.36; P=0.08; I2=0%), SAEs (OR, 0.45; 95% CI, 0.08–2.54; P=0.37; I2=0%), ketoacidosis (OR, 0.33; 95% CI, 0.01–8.37; P=0.50), urinary tract infections (OR, 2.34; 95% CI, 0.44–12.50; P=0.32; I2=0%), and severe hypoglycemia (OR, 4.47; 95% CI, 0.21–96.40; P=0.34) were comparable among the SGLTi group and placebo. Compared to placebo, T2DM children receiving SGLTi had significantly lower glycosylated hemoglobin at 24–26 weeks (mean difference [MD], -0.79%; 95% CI, -1.33 to -0.26; P=0.004; I2=0%). In T1DM children, β-hydroxybutyrate levels were significantly higher in the SGLTi group than the placebo group (MD, 0.11 mmol/L; 95% CI, 0.05–0.17; P=0.0005; I2=53%). In T1DM, there was not a single report of an SAE, ketoacidosis, or severe hypoglycemia in either the placebo or treatment groups, but time-in-range was considerably greater in the SGLT2i group than the placebo group (68%±6% vs. 50%±13%, P<0.001).
Conclusion
SGLT2i use in children and young adults appears to be both safe and tolerable based on our meta-analyses and review of the literature.
Keyword
Figure
Reference
-
References
1. U.S. Food and Drug Agency (FDA). FDA approves new class of medicines to treat pediatric type 2 diabetes [Internet]. Silver Spring (MD): FDA;2023. [cited 2023 Jun 28]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-class-medicines-treat-pediatric-type-2-diabetes.2. Cioana M, Deng J, Nadarajah A, Hou M, Qiu Y, Chen SSJ, et al. The prevalence of obesity among children with type 2 diabetes: a systematic review and meta-analysis. JAMA Netw Open. 2022; 5:e2247186.3. He QX, Zhao L, Tong JS, Liang XY, Li RN, Zhang P, et al. The impact of obesity epidemic on type 2 diabetes in children and adolescents: a systematic review and meta-analysis. Prim Care Diabetes. 2022; 16:736–44.
Article4. Phillip M, Mathieu C, Lind M, Araki E, di Bartolo P, Bergenstal R, et al. Long-term efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: pooled 52-week outcomes from the DEPICT-1 and -2 studies. Diabetes Obes Metab. 2021; 23:549–60.5. Mathieu C, Dandona P, Birkenfeld AL, Hansen TK, Iqbal N, Xu J, et al. Benefit/risk profile of dapagliflozin 5 mg in the DEPICT-1 and -2 trials in individuals with type 1 diabetes and body mass index ≥27 kg/m2. Diabetes Obes Metab. 2020; 22:2151–60.6. Dos Santos SS, Ramaldes LA, Gabbay MAL, Moises RCS, Dib SA. Use of a sodium-glucose cotransporter 2 inhibitor, empagliflozin, in a patient with Rabson-Mendenhall syndrome. Horm Res Paediatr. 2021; 94:313–6.
Article7. Galderisi A, Tamborlane W, Taylor SI, Attia N, Moretti C, Barbetti F. SGLT2i improves glycemic control in patients with congenital severe insulin resistance. Pediatrics. 2022; 150:e2021055671.
Article8. Grünert SC, Derks TGJ, Adrian K, Al-Thihli K, Ballhausen D, Bidiuk J, et al. Efficacy and safety of empagliflozin in glycogen storage disease type Ib: Data from an international questionnaire. Genet Med Off J Am Coll Med Genet. 2022; 24:1781–8.9. Candler T, McGregor D, Narayan K, Moudiotis C, Burren CP. Improvement in glycaemic parameters using SGLT-2 inhibitor and GLP-1 agonist in combination in an adolescent with diabetes mellitus and Prader-Willi syndrome: a case report. J Pediatr Endocrinol Metab. 2020; 33:951–5.
Article10. Newland DM, Law YM, Albers EL, Friedland-Little JM, Ahmed H, Kemna MS, et al. Early clinical experience with dapagliflozin in children with heart failure. Pediatr Cardiol. 2023; 44:146–52.
Article11. Cui JY, Liu JJ, Fang XY, Chen J, Zhai YH, Xu H, et al. [Short-term efficacy of dapagliflozin in children with hereditary proteinuric kidney disease]. Zhonghua Er Ke Za Zhi Chin J Pediatr. 2023; 61:164–8.12. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.
Article13. Dutta D, Bhattacharya S, Surana V, Aggarwal S, Singla R, Khandelwal D, et al. Efficacy and safety of saroglitazar in managing hypertriglyceridemia in type-2 diabetes: a meta-analysis. Diabetes Metab Syndr. 2020; 14:1759–68.
Article14. Dutta D, Agarwal A, Maisnam I, Singla R, Khandelwal D, Sharma M. Efficacy and safety of the novel dipeptidyl peptidase-4 inhibitor gemigliptin in the management of type 2 diabetes: a meta-analysis. Endocrinol Metab (Seoul). 2021; 36:374–87.
Article15. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009; 339:b2700.
Article16. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008; 336:924–6.
Article17. Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ. Publication and related biases. Health Technol Assess Winch Engl. 2000; 4:1–115.
Article18. Laffel LM, Danne T, Klingensmith GJ, Tamborlane WV, Willi S, Zeitler P, et al. Efficacy and safety of the SGLT2 inhibitor empagliflozin versus placebo and the DPP-4 inhibitor linagliptin versus placebo in young people with type 2 diabetes (DINAMO): a multicentre, randomised, double-blind, parallel group, phase 3 trial. Lancet Diabetes Endocrinol. 2023; 11:169–81.19. Tamborlane WV, Laffel LM, Shehadeh N, Isganaitis E, Van Name M, Ratnayake J, et al. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinol. 2022; 10:341–50.
Article20. Biester T, Muller I, von dem Berge T, Atlas E, Nimri R, Phillip M, et al. Add-on therapy with dapagliflozin under full closed loop control improves time in range in adolescents and young adults with type 1 diabetes: The DAPADream study. Diabetes Obes Metab. 2021; 23:599–608.
Article21. Biester T, Aschemeier B, Fath M, Frey M, Scheerer MF, Kordonouri O, et al. Effects of dapagliflozin on insulin-requirement, glucose excretion and β-hydroxybutyrate levels are not related to baseline HbA1c in youth with type 1 diabetes. Diabetes Obes Metab. 2017; 19:1635–9.22. Laffel LMB, Tamborlane WV, Yver A, Simons G, Wu J, Nock V, et al. Pharmacokinetic and pharmacodynamic profile of the sodium-glucose co-transporter-2 inhibitor empagliflozin in young people with Type 2 diabetes: a randomized trial. Diabet Med J Br Diabet Assoc. 2018; 35:1096–104.
Article23. Tirucherai GS, LaCreta F, Ismat FA, Tang W, Boulton DW. Pharmacokinetics and pharmacodynamics of dapagliflozin in children and adolescents with type 2 diabetes mellitus. Diabetes Obes Metab. 2016; 18:678–84.
Article24. Halligan RK, Dalton RN, Turner C, Lewis KA, Mundy HR. Understanding the role of SGLT2 inhibitors in glycogen storage disease type Ib: the experience of one UK centre. Orphanet J Rare Dis. 2022; 17:195.
Article25. Urakami T, Yoshida K, Suzuki J. Efficacy of low-dose dapagliflozin in young people with type 1 diabetes. Intern Med. 2023; 62:177–86.
Article26. Kumar P, Srivastava S, Mishra PS, Mooss ET. Prevalence of pre-diabetes/type 2 diabetes among adolescents (10-19 years) and its association with different measures of overweight/obesity in India: a gendered perspective. BMC Endocr Disord. 2021; 21:146.27. Wu S, He Y, Wu Y, Ji Y, Hou L, Liu X, et al. Comparative efficacy and safety of glucose-lowering drugs in children and adolescents with type 2 diabetes: a systematic review and network meta-analysis. Front Endocrinol. 2022; 13:897776.28. Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, et al. Heart failure, saxagliptin, and diabetes mel litus : obs er v at ions f rom t he S AVOR- TI MI 53 randomized trial. Circulation. 2015; 132:e198.
Article29. Ferrannini E, Mark M, Mayoux E. CV Protection in the EMPA-REG OUTCOME Trial: A "Thrifty Substrate" Hypothesis. Diabetes Care. 2016; 39:1108–14.
Article30. Urakami T. Treatment strategy for children and adolescents with type 2 diabetes-based on ISPAD Clinical Practice Consensus Guidelines 2022. Clin Pediatr Endocrinol. 2023; 32:125–36.
Article31. American Diabetes Association Professional Practice Committee. 14. Children and Adolescents: Standards of Care in Diabetes-2024. Diabetes Care. 2024; 47 Suppl 1:S258–81.32. Peña AS, Curran JA, Fuery M, George C, Jefferies CA, Lobley K, et al. Screening, assessment and management of type 2 diabetes mellitus in children and adolescents: Australasian Paediatric Endocrine Group guidelines. Med J Aust. 2020; 213:30–43.
Article33. Ong Lopez AMC, Pajimna JAT. Efficacy of sodium glucose cotransporter 2 inhibitors on hepatic fibrosis and steatosis in non-alcoholic fatty liver disease: an updated systematic review and meta-analysis. Sci Rep. 2024; 14:2122.
Article34. Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020; 17:761–72.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Emerging Safety Issues of Dipeptidyl Peptidase-4 Inhibitors and Sodium Glucose Cotransporter 2 Inhibitors: How to Interpret and Apply in Clinical Practice
- The Side Effects of Sodium Glucose Cotransporter 2 (SGLT2) Inhibitor
- Glucose Lowering Effect of SGLT2 Inhibitors: A Review of Clinical Studies
- Sodium-Glucose Cotransporter 2 Inhibitors for People with Type 1 Diabetes
- SGLT2 Inhibitors and Ketoacidosis: Pathophysiology and Management