Cancer Res Treat.
2024 Apr;56(2):688-696. 10.4143/crt.2023.1049.
Assessment of Bone Marrow Involvement in Extranodal NK/T-Cell Lymphoma: Positron Emission Tomography versus Bone Marrow Biopsy, and the Significance of Minimal Involvement by EBV+ Cells (KROG 18-09)
- Affiliations
-
- 1Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Radiation Oncology, Gachon University Gil Hospital, Incheon, Korea
- 3Department of Radiation Oncology, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- 4Department of Radiation Oncology, Inha University Hospital, Inha University School of Medicine, Incheon, Korea
- 5Department of Radiation Oncology, Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea
- 6Department of Radiation Oncology, Seoul National University Bundang Hospital, Seongnam, Korea
- KMID: 2554358
- DOI: http://doi.org/10.4143/crt.2023.1049
Abstract
- Purpose
This study aims to investigate the diagnostic significance of positron emission tomography/computed tomography (PET/CT) in assessing bone marrow (BM) involvement through a comparison of PET/CT findings with BM biopsy in extranodal natural killer/T-cell lymphoma.
Materials and Methods
The medical records of 193 patients were retrospectively reviewed. Patients were categorized as having early-stage (PET-ES) or advanced-stage (PET-AS) disease based on PET/CT results. The BM involvement was classified into three groups according to BM biopsy: gross BM involvement, minimal BM involvement (defined as the presence of a limited number of Epstein-Barr virus–positive cells in BM), and no involvement. Calculations of the accuracy of PET/CT in detecting BM involvement and analysis of the clinical outcomes (progression-free survival [PFS] and overall survival [OS]) according to the BM biopsy status were performed.
Results
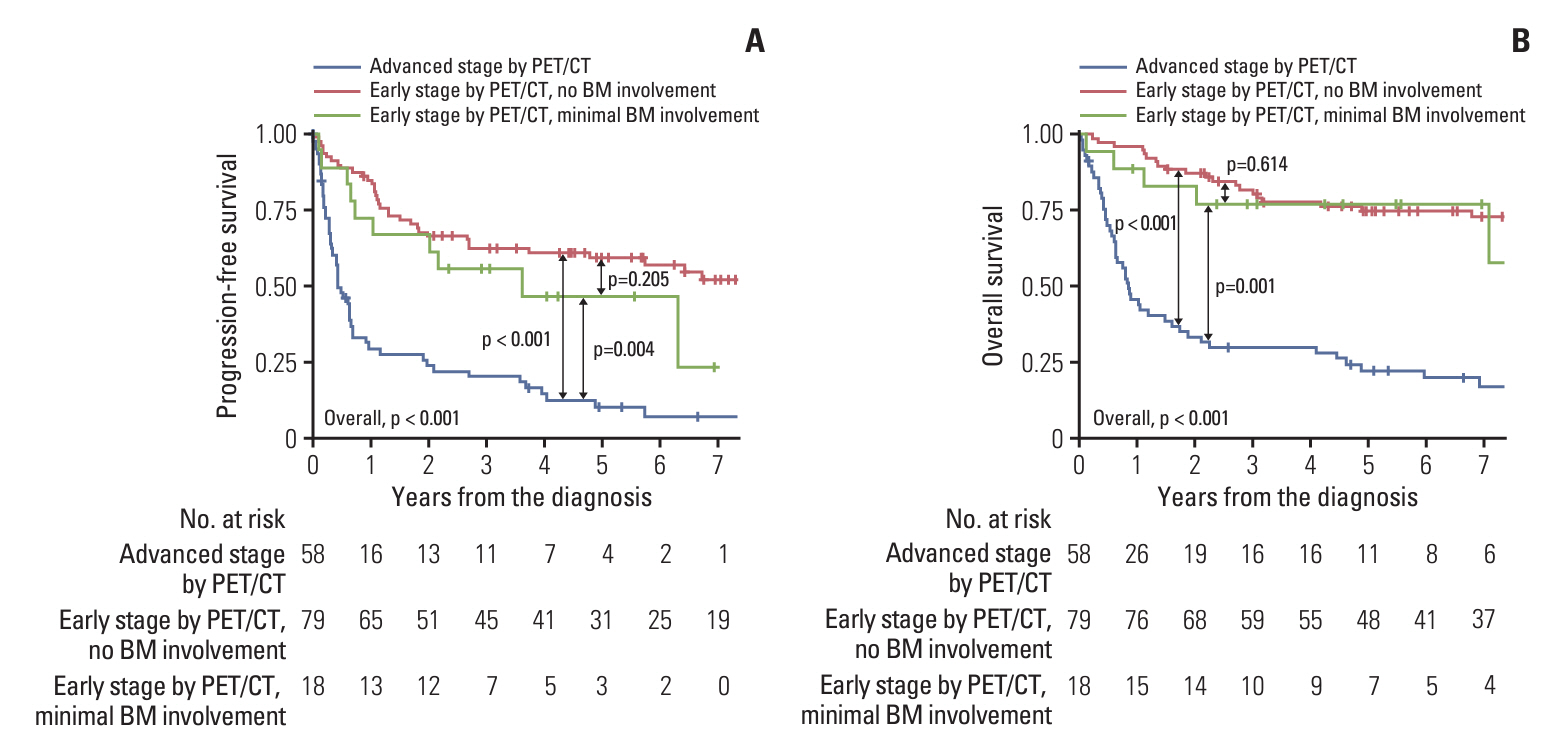
PET/CT exhibited a sensitivity of 64.7% and a specificity of 96.0% in detecting gross BM involvement. For detecting any (both gross and minimal) BM involvement, the sensitivity was 30.4%, while the specificity was 99.0%. Only one patient (0.7%) demonstrated gross BM involvement among the PET-ES group. Survival outcomes of the PET-ES group with minimal BM involvement (3-year PFS, 55.6%; OS, 77.0%) were closer to those of the PET-ES group with no BM involvement (3-year PFS, 62.2%; OS, 80.6%) than to those of the PET-AS group (3-year PFS, 20.1%; OS, 29.9%).
Conclusion
PET/CT exhibits high specificity, but moderate and low sensitivity in detecting gross and minimal BM involvement, respectively. The clinical significance of minimal BM involvement for patients in the PET-ES group may be limited.
Figure
Reference
-
References
1. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IB, Berti E, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022; 36:1720–48.2. Tse E, Kwong YL. The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol. 2017; 10:85.
Article3. Perry AM, Diebold J, Nathwani BN, MacLennan KA, Muller-Hermelink HK, Bast M, et al. Non-Hodgkin lymphoma in the developing world: review of 4539 cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica. 2016; 101:1244–50.
Article4. Tse E, Zhao WL, Xiong J, Kwong YL. How we treat NK/T-cell lymphomas. J Hematol Oncol. 2022; 15:74.
Article5. Yang Y, Cao JZ, Lan SM, Wu JX, Wu T, Zhu SY, et al. Association of improved locoregional control with prolonged survival in early-stage extranodal nasal-type natural killer/T-cell lymphoma. JAMA Oncol. 2017; 3:83–91.
Article6. Deng XW, Wu JX, Wu T, Zhu SY, Shi M, Su H, et al. Radiotherapy is essential after complete response to asparaginase-containing chemotherapy in early-stage extranodal nasal-type NK/T-cell lymphoma: a multicenter study from the China Lymphoma Collaborative Group (CLCG). Radiother Oncol. 2018; 129:3–9.
Article7. Fujiwara H, Maeda Y, Nawa Y, Yamakura M, Ennishi D, Miyazaki Y, et al. The utility of positron emission tomography/computed tomography in the staging of extranodal natural killer/T-cell lymphoma. Eur J Haematol. 2011; 87:123–9.
Article8. Moon SH, Cho SK, Kim WS, Kim SJ, Chan Ahn Y, Choe YS, et al. The role of 18F-FDG PET/CT for initial staging of nasal type natural killer/T-cell lymphoma: a comparison with conventional staging methods. J Nucl Med. 2013; 54:1039–44.
Article9. Qi SN, Li YX, Specht L, Oguchi M, Tsang R, Ng A, et al. Modern radiation therapy for extranodal nasal-type NK/T-cell lymphoma: risk-adapted therapy, target volume, and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2021; 110:1064–81.
Article10. NCCN Clinical Practice Guidelines in Oncology: T-cell lymphomas, version 1.2023 [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;2023. [cited 2023 Dec 20]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf.11. Tilly H, Gomes da Silva M, Vitolo U, Jack A, Meignan M, Lopez-Guillermo A, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015; 26 Suppl 5:v116–25.
Article12. Eichenauer DA, Aleman BM, Andre M, Federico M, Hutchings M, Illidge T, et al. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018; 29:iv19–29.
Article13. Zhou Z, Chen C, Li X, Li Z, Zhang X, Chang Y, et al. Evaluation of bone marrow involvement in extranodal NK/T cell lymphoma by FDG-PET/CT. Ann Hematol. 2015; 94:963–7.
Article14. Wang Y, Xie L, Tian R, Deng Y, Zhang W, Zou L, et al. PET/CT-based bone-marrow assessment shows potential in replacing routine bone-marrow biopsy in part of patients newly diagnosed with extranodal natural killer/T-cell lymphoma. J Cancer Res Clin Oncol. 2019; 145:2529–39.
Article15. Koh Y, Lee JM, Woo GU, Paeng JC, Youk J, Yoon SS, et al. FDG PET for evaluation of bone marrow status in T-cell lymphoma. Clin Nucl Med. 2019; 44:4–10.
Article16. Yang C, Wu W, Zhou H, Zhao S, Tian R, Xiang M, et al. (18) F-FDG PET/CT plays a limited role in replacing bone marrow biopsy for newly diagnosed advanced-stage patients with extranodal natural killer/T-cell lymphoma. Front Oncol. 2022; 12:894804.17. Yang Y, Wang JJ, Zhao RZ, Huang C, Shi GQ, Zheng H, et al. The value of routine bone marrow examination in patients with extranodal NK/T-cell lymphoma staged with PET/CT. Cancer. 2022; 128:3943–50.
Article18. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32:3059–68.
Article19. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol. 1995; 57:289–300.
Article20. Lee J, Suh C, Huh J, Jun HJ, Kim K, Jung C, et al. Effect of positive bone marrow EBV in situ hybridization in staging and survival of localized extranodal natural killer/T-cell lymphoma, nasal-type. Clin Cancer Res. 2007; 13:3250–4.21. Huang WT, Chang KC, Huang GC, Hsiao JR, Chen HH, Chuang SS, et al. Bone marrow that is positive for Epstein-Barr virus encoded RNA-1 by in situ hybridization is related with a poor prognosis in patients with extranodal natural killer/T-cell lymphoma, nasal type. Haematologica. 2005; 90:1063–9.22. Ito Y, Makita S, Maeshima AM, Hatta S, Yuda S, Suzuki T, et al. EBV-encoded RNA1-positive cells in the bone marrow specimens of patients with EBV-negative lymphomas and sarcomas. Pathol Int. 2019; 69:392–7.
Article23. Farrell D, Nael A, Law T, Zhao X, Weiss LM, Rezk SA. Epstein-Barr virus incidental expression in bone marrow cells: a study of 230 consecutive bone marrow biopsy samples. Hum Pathol. 2019; 88:60–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone Marrow is Involved in Less than 10% of Patients with Nasal-Type NK/T Cell Lymphoma at Initial Diagnosis
- Detection of bone marrow involvement with FDG PET/CT in patients with newly diagnosed lymphoma
- Persistent Anemia in a Patient with Diffuse Large B Cell Lymphoma: Pure Red Cell Aplasia Associated with Latent Epstein-Barr Virus Infection in Bone Marrow
- A Case of Monoclonal Gammopathy in Extranodal Marginal Zone B-cell Lymphoma of the Small Intestine
- A Case of Hodgkin`s Lymphoma with Bone Marrow Involvement Showing Severe Osteosclerosis