Cancer Res Treat.
2024 Apr;56(2):642-651. 10.4143/crt.2023.999.
Tandem High-Dose Chemotherapy Increases the Risk of Secondary Malignant Neoplasm in Pediatric Solid Tumors
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Pediatrics, CHA Gangnam Medical Center, CHA University School of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2554353
- DOI: http://doi.org/10.4143/crt.2023.999
Abstract
- Purpose
This study aimed to investigate the incidence and risk factors for secondary malignant neoplasms (SMN) in pediatric solid tumors, focusing on the effects of tandem high-dose chemotherapy (HDCT).
Materials and Methods
Patients (aged < 19 years) diagnosed with or treated for pediatric solid tumors between 1994 and 2014 were retrospectively analyzed. The cumulative incidence of SMN was estimated using competing risk methods by considering death as a competing risk.
Results
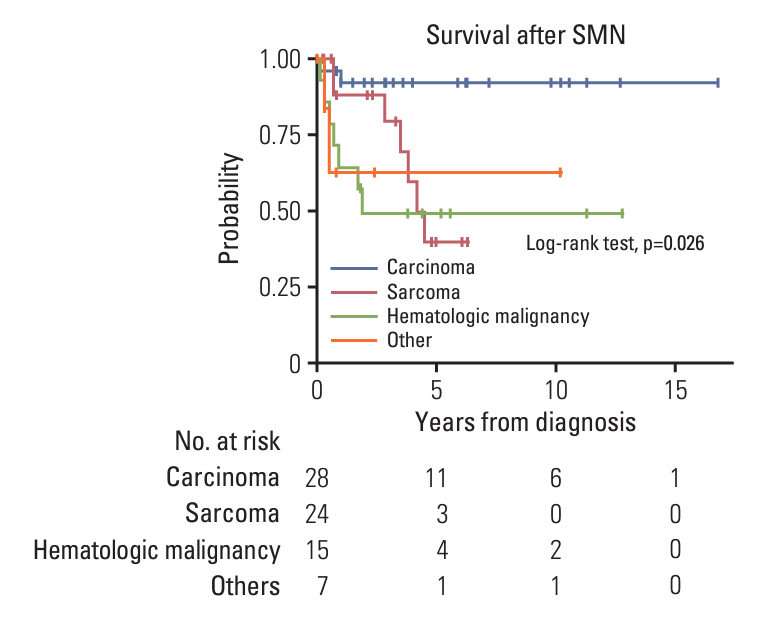
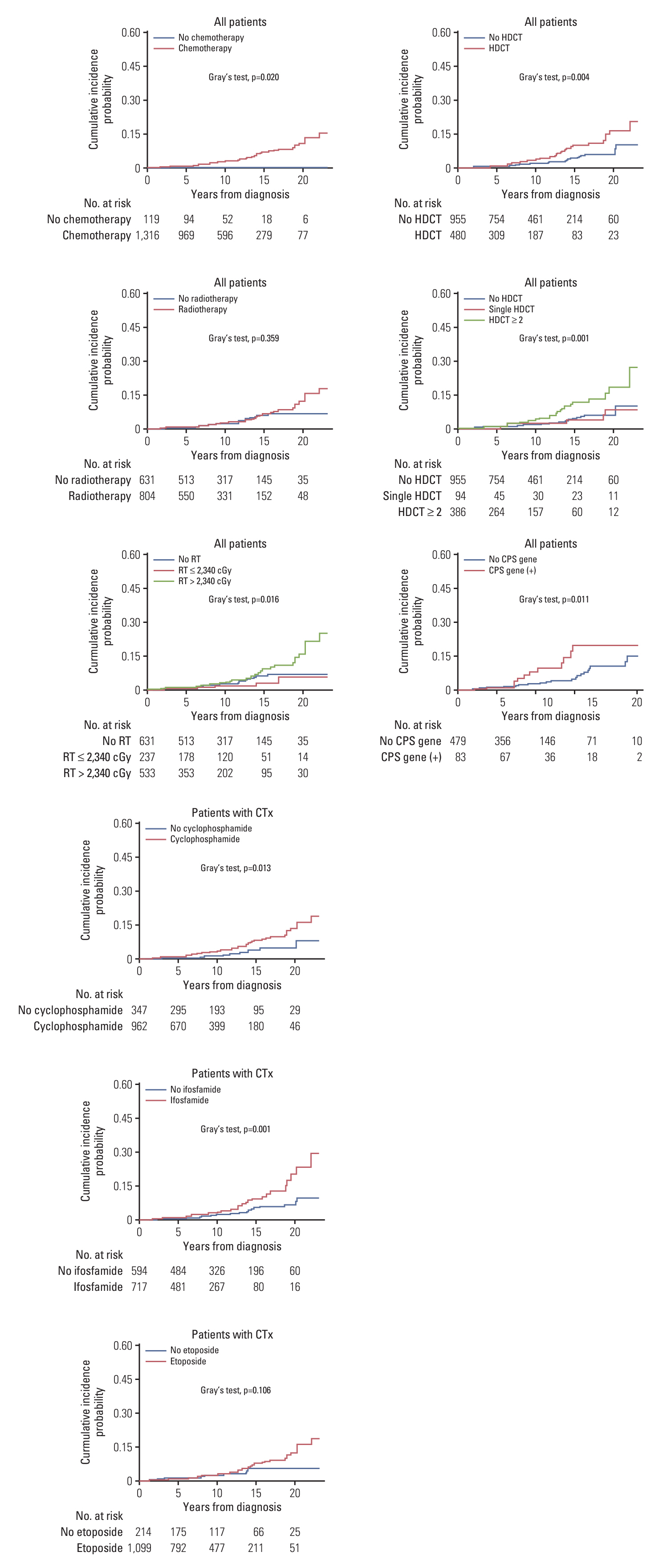
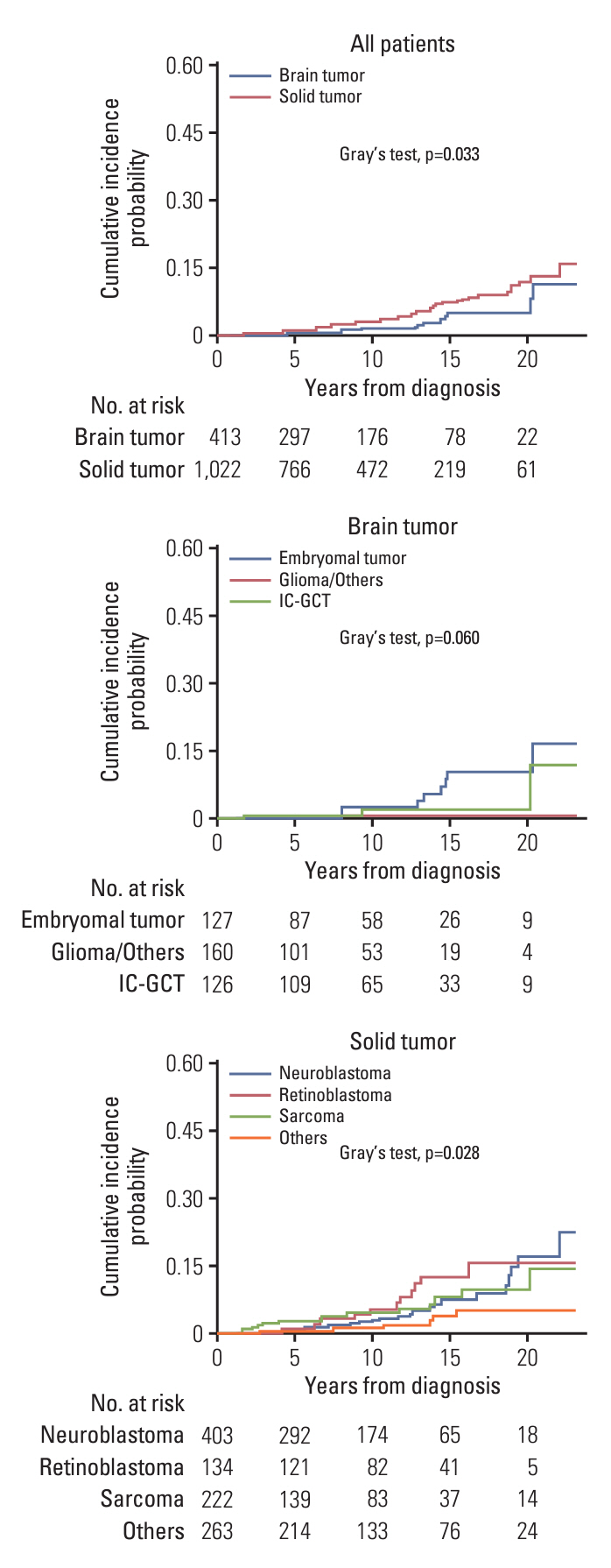
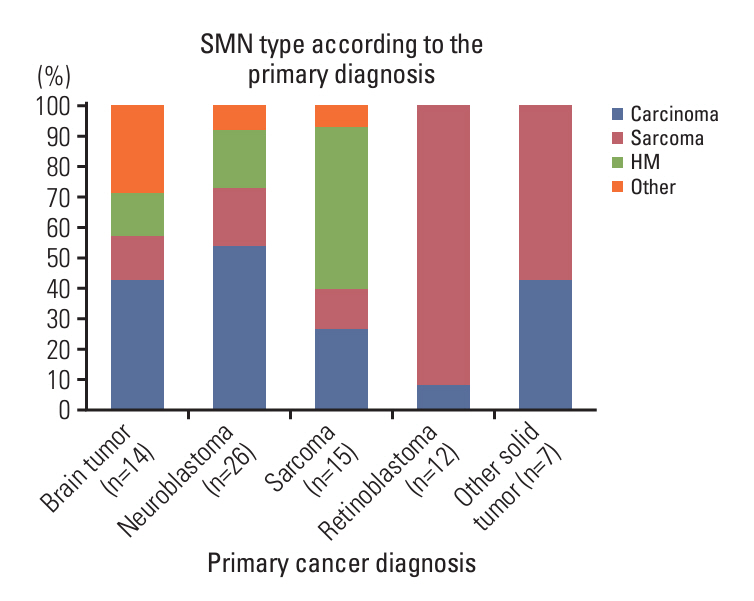
A total of 1,435 patients (413 with brain tumors and 1,022 with extracranial solid tumors) were enrolled. Seventy-one patients developed 74 SMNs, with a 10-year and 20-year cumulative incidence of 2.680±0.002% and 10.193±0.024%, respectively. The types of SMN included carcinoma in 28 (37.8%), sarcoma in 24 (32.4%), and hematologic malignancy in 15 (20.3%) cases. Osteosarcoma and thyroid carcinoma were the most frequently diagnosed tumors. Multivariate analysis showed that radiotherapy (RT) > 2, 340 cGy, and tandem HDCT were significant risk factors for SMN development. The SMN types varied according to the primary tumor type; carcinoma was the most frequent SMN in brain tumors and neuroblastoma, whereas hematologic malignancy and sarcomas developed more frequently in patients with sarcoma and retinoblastoma, respectively.
Conclusion
The cumulative incidence of SMN in pediatric patients with solid tumors was considerably high, especially in patients who underwent tandem HDCT or in those who received RT > 2,340 cGy. Therefore, the treatment intensity should be optimized based on individual risk assessment and the long-term follow-up of pediatric cancer survivors.
Figure
Reference
-
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022; 72:7–33.
Article2. Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008; 100:1368–79.
Article3. Kletzel M, Katzenstein HM, Haut PR, Yu AL, Morgan E, Reynolds M, et al. Treatment of high-risk neuroblastoma with triple-tandem high-dose therapy and stem-cell rescue: results of the Chicago Pilot II Study. J Clin Oncol. 2002; 20:2284–92.
Article4. Matthay KK. Intensification of therapy using hematopoietic stem-cell support for high-risk neuroblastoma. Pediatr Transplant. 1999; 3 Suppl 1:72–7.
Article5. Park JR, Kreissman SG, London WB, Naranjo A, Cohn SL, Hogarty MD, et al. Effect of tandem autologous stem cell transplant vs single transplant on event-free survival in patients with high-risk neuroblastoma: a randomized clinical trial. JAMA. 2019; 322:746–55.
Article6. Lee JW, Lee S, Cho HW, Ma Y, Yoo KH, Sung KW, et al. Incorporation of high-dose (131)I-metaiodobenzylguanidine treatment into tandem high-dose chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma: results of the SMC NB-2009 study. J Hematol Oncol. 2017; 10:108.
Article7. Sung KW, Lim DH, Son MH, Lee SH, Yoo KH, Koo HH, et al. Reduced-dose craniospinal radiotherapy followed by tandem high-dose chemotherapy and autologous stem cell transplantation in patients with high-risk medulloblastoma. Neuro Oncol. 2013; 15:352–9.
Article8. Sung KW, Lim DH, Yi ES, Choi YB, Lee JW, Yoo KH, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation for atypical teratoid/rhabdoid tumor. Cancer Res Treat. 2016; 48:1408–19.
Article9. Sung KW, Son MH, Lee SH, Yoo KH, Koo HH, Kim JY, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation in patients with high-risk neuroblastoma: results of SMC NB-2004 study. Bone Marrow Transplant. 2013; 48:68–73.
Article10. Ma Y, Lim DH, Cho H, Lee JW, Sung KW, Yoo KH, et al. Tandem high-dose chemotherapy without craniospinal irradiation in treatment of non-metastatic malignant brain tumors in very young children. J Korean Med Sci. 2020; 35:e405.
Article11. Ju HY, Moon EK, Lim J, Park BK, Shin HY, Won YJ, et al. Second malignant neoplasms after childhood cancer: A nationwide population-based study in Korea. PLoS One. 2018; 13:e0207243.
Article12. Hammal DM, Bell CL, Craft AW, Parker L. Second primary tumors in children and young adults in the North of England (1968-99). Pediatr Blood Cancer. 2005; 45:155–61.
Article13. Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007; 13:559–65.
Article14. Laska E, Meisner M, Wanderling J. A maximally selected test of symmetry about zero. Stat Med. 2012; 31:3178–91.
Article15. Neglia JP, Friedman DL, Yasui Y, Mertens AC, Hammond S, Stovall M, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001; 93:618–29.
Article16. Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010; 102:1083–95.17. Ishida Y, Qiu D, Maeda M, Fujimoto J, Kigasawa H, Kobayashi R, et al. Secondary cancers after a childhood cancer diagnosis: a nationwide hospital-based retrospective cohort study in Japan. Int J Clin Oncol. 2016; 21:506–16.
Article18. Baker KS, DeFor TE, Burns LJ, Ramsay NK, Neglia JP, Robison LL. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003; 21:1352–8.
Article19. Danner-Koptik KE, Majhail NS, Brazauskas R, Wang Z, Buchbinder D, Cahn JY, et al. Second malignancies after autologous hematopoietic cell transplantation in children. Bone Marrow Transplant. 2013; 48:363–8.
Article20. Applebaum MA, Vaksman Z, Lee SM, Hungate EA, Henderson TO, London WB, et al. Neuroblastoma survivors are at increased risk for second malignancies: a report from the International Neuroblastoma Risk Group Project. Eur J Cancer. 2017; 72:177–85.
Article21. Forrest DL, Nevill TJ, Naiman SC, Le A, Brockington DA, Barnett MJ, et al. Second malignancy following high-dose therapy and autologous stem cell transplantation: incidence and risk factor analysis. Bone Marrow Transplant. 2003; 32:915–23.
Article22. Vaxman I, Ram R, Gafter-Gvili A, Vidal L, Yeshurun M, Lahav M, et al. Secondary malignancies following high dose therapy and autologous hematopoietic cell transplantation-systematic review and meta-analysis. Bone Marrow Transplant. 2015; 50:706–14.
Article23. Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006; 7:813–20.
Article24. Goldsby R, Burke C, Nagarajan R, Zhou T, Chen Z, Marina N, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children’s Oncology Group cohort. Cancer. 2008; 113:2597–604.
Article25. Kaatsch P, Reinisch I, Spix C, Berthold F, Janka-Schaub G, Mergenthaler A, et al. Case-control study on the therapy of childhood cancer and the occurrence of second malignant neoplasms in Germany. Cancer Causes Control. 2009; 20:965–80.
Article26. Kuttesch JF Jr, Wexler LH, Marcus RB, Fairclough D, Weaver-McClure L, White M, et al. Second malignancies after Ewing’s sarcoma: radiation dose-dependency of secondary sarcomas. J Clin Oncol. 1996; 14:2818–25.
Article27. Constine LS, Tarbell N, Hudson MM, Schwartz C, Fisher SG, Muhs AG, et al. Subsequent malignancies in children treated for Hodgkin’s disease: associations with gender and radiation dose. Int J Radiat Oncol Biol Phys. 2008; 72:24–33.
Article28. Lee JW, Lim DH, Sung KW, Cho HW, Ju HY, Yoo KH, et al. Induction chemotherapy reduces radiation therapy dose and volume in the treatment of intracranial germinoma: results of the SMC-G13 trial. Int J Radiat Oncol Biol Phys. 2020; 108:649–56.
Article29. Lim DH, Yoo KH, Lee NH, Lee SH, Sung KW, Koo HH, et al. Intensive chemotherapy followed by reduced-dose radiotherapy for biopsy-proven CNS germinoma with elevated beta-human chorionic gonadotropin. J Neurooncol. 2014; 117:279–85.
Article30. Hisada M, Garber JE, Fung CY, Fraumeni JF Jr, Li FP. Multiple primary cancers in families with Li-Fraumeni syndrome. J Natl Cancer Inst. 1998; 90:606–11.
Article31. Waespe N, Belle FN, Redmond S, Schindera C, Spycher BD, Rossler J, et al. Cancer predisposition syndromes as a risk factor for early second primary neoplasms after childhood cancer - A national cohort study. Eur J Cancer. 2021; 145:71–80.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Cardiac Function in Children with Solid Tumors, who Underwent Tandem High-Dose Chemotherapy and Autologous Stem Cell Transplantation
- Hematologic Recovery after Tandem High-Dose Chemotherapy and Autologous Stem Cell Transplantation in Children with High-Risk Solid Tumors
- Tandem High-dose Chemotherapy and Autologous Stem Cell Transplantation in Children with Brain Tumors : Review of Single Center Experience
- Ifosfamide in the pediatric malignant solid tumors
- Tandem High-dose Chemotherapy without Craniospinal Irradiation in Treatment of Non-metastatic Malignant Brain Tumors in Very Young Children