Cancer Res Treat.
2024 Apr;56(2):557-566. 10.4143/crt.2023.1025.
PD-L1 (SP142) Expression in Primary and Recurrent/Metastatic Triple-Negative Breast Cancers and Its Clinicopathological Significance
- Affiliations
-
- 1Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 2Division of Hematology and Medical Oncology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- KMID: 2554345
- DOI: http://doi.org/10.4143/crt.2023.1025
Abstract
- Purpose
The programmed death-ligand 1 (PD-L1) SP142 assay identifies patients with triple-negative breast cancer (TNBC) who are most likely to respond to the anti–PD-L1 agent atezolizumab. We aimed to compare PD-L1 (SP142) expression between primary and recurrent/metastatic TNBCs and elucidate the clinicopathological features associated with its expression.
Materials and Methods
Primary and recurrent/metastatic TNBCs tested with PD-L1 (SP142) were collected, and clinicopathological information of these cases was obtained through a review of slides and medical records.
Results
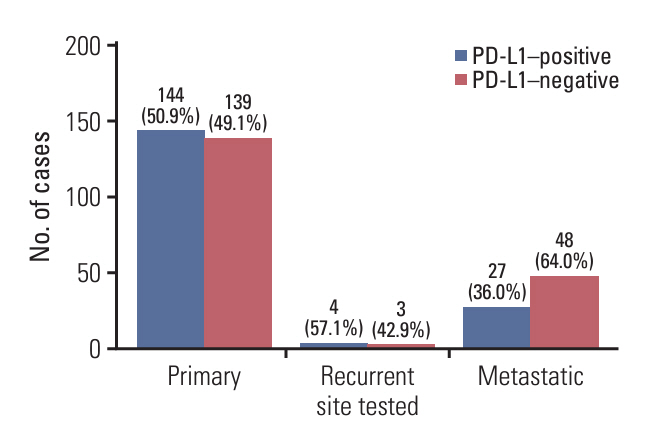
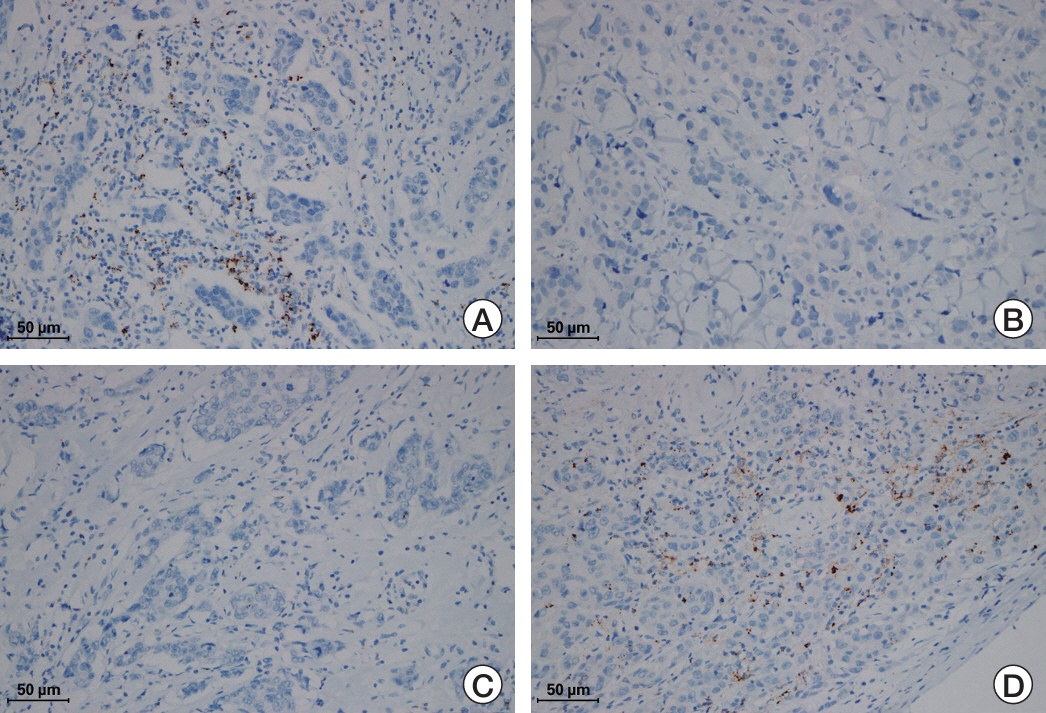
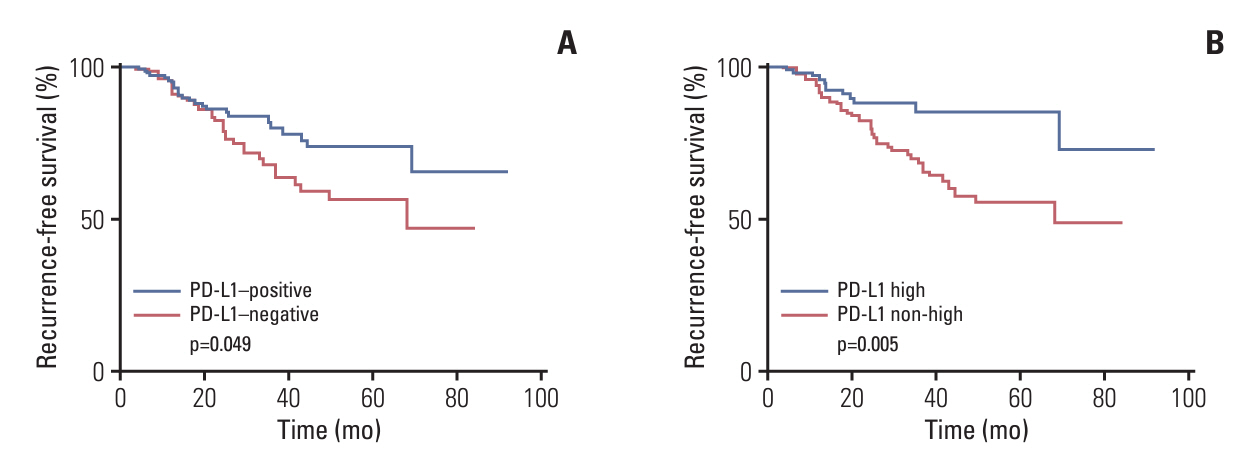
PD-L1 (SP142) positivity was observed in 50.9% (144/283) of primary tumors and 37.8% (31/82) of recurrent/metastatic TNBCs with a significant difference. Recurrent or metastatic sites were associated with PD-L1 positivity, with high PD-L1 positivity in the lung, breast, and soft tissues, and low positivity in the bone, skin, liver, and brain. When comparing PD-L1 expression between primary and matched recurrent/metastatic TNBCs using 55 paired samples, 20 cases (36.4%) showed discordance; 10 cases revealed positive conversion, and another 10 cases revealed negative conversion during metastatic progression. In primary TNBCs, PD-L1 expression was associated with a higher histologic grade, lower T category, pushing border, and higher tumor-infiltrating lymphocyte infiltration. In survival analyses, PD-L1 positivity, especially high positivity, was found to be associated with favorable prognosis of patients.
Conclusion
PD-L1 (SP142) expression was lower in recurrent/metastatic TNBCs, and substantial cases showed discordance in its expression between primary and recurrent/metastatic sites, suggesting that multiple sites may need to be tested for PD-L1 (SP142) when considering atezolizumab therapy. PD-L1 (SP142)–positive TNBCs seems to be associated with favorable clinical outcomes.
Keyword
Figure
Reference
-
References
1. Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001; 98:10869–74.
Article2. Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017; 377:523–33.
Article3. Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018; 379:753–63.
Article4. Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013; 499:214–8.
Article5. Luen S, Virassamy B, Savas P, Salgado R, Loi S. The genomic landscape of breast cancer and its interaction with host immunity. Breast. 2016; 29:241–50.
Article6. Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014; 2:361–70.
Article7. Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013; 31:860–7.
Article8. Tan Q, Yin S, Zhou D, Chi Y, Man X, Li H. Potential predictive and prognostic value of biomarkers related to immune checkpoint inhibitor therapy of triple-negative breast cancer. Front Oncol. 2022; 12:779786.
Article9. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000; 192:1027–34.
Article10. Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012; 366:2517–9.
Article11. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020; 396:1817–28.12. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018; 379:2108–21.
Article13. Hoda RS, Brogi E, Dos Anjos CH, Grabenstetter A, Ventura K, Patil S, et al. Clinical and pathologic features associated with PD-L1 (SP142) expression in stromal tumor-infiltrating immune cells of triple-negative breast carcinoma. Mod Pathol. 2020; 33:2221–32.
Article14. Rozenblit M, Huang R, Danziger N, Hegde P, Alexander B, Ramkissoon S, et al. Comparison of PD-L1 protein expression between primary tumors and metastatic lesions in triple negative breast cancers. J Immunother Cancer. 2020; 8:e001558.
Article15. Manson QF, Schrijver W, Ter Hoeve ND, Moelans CB, van Diest PJ. Frequent discordance in PD-1 and PD-L1 expression between primary breast tumors and their matched distant metastases. Clin Exp Metastasis. 2019; 36:29–37.
Article16. Tawfik O, Kimler BF, Karnik T, Shehata P. Clinicopathological correlation of PD-L1 expression in primary and metastatic breast cancer and infiltrating immune cells. Hum Pathol. 2018; 80:170–8.
Article17. Li Y, Vennapusa B, Chang CW, Tran D, Nakamura R, Sumiyoshi T, et al. Prevalence study of PD-L1 SP142 assay in metastatic triple-negative breast cancer. Appl Immunohistochem Mol Morphol. 2021; 29:258–64.
Article18. Miyakoshi J, Yazaki S, Shimoi T, Onishi M, Saito A, Kita S, et al. Discordance in PD-L1 expression using 22C3 and SP142 assays between primary and metastatic triple-negative breast cancer. Virchows Arch. 2023; 483:855–63.
Article19. Ogiya R, Niikura N, Kumaki N, Bianchini G, Kitano S, Iwamoto T, et al. Comparison of tumor-infiltrating lymphocytes between primary and metastatic tumors in breast cancer patients. Cancer Sci. 2016; 107:1730–5.
Article20. Cimino-Mathews A, Ye X, Meeker A, Argani P, Emens LA. Metastatic triple-negative breast cancers at first relapse have fewer tumor-infiltrating lymphocytes than their matched primary breast tumors: a pilot study. Hum Pathol. 2013; 44:2055–63.
Article21. Sobottka B, Pestalozzi B, Fink D, Moch H, Varga Z. Similar lymphocytic infiltration pattern in primary breast cancer and their corresponding distant metastases. Oncoimmunology. 2016; 5:e1153208.
Article22. Baek SH, Kim JH, Bae SJ, Ji JH, Lee Y, Jeong J, et al. SP142 PD-L1 assays in multiple samples from the same patients with early or advanced triple-negative breast cancer. Cancers (Basel). 2022; 14:3042.
Article23. Dieci MV, Tsvetkova V, Orvieto E, Piacentini F, Ficarra G, Griguolo G, et al. Immune characterization of breast cancer metastases: prognostic implications. Breast Cancer Res. 2018; 20:62.
Article24. Boman C, Zerdes I, Martensson K, Bergh J, Foukakis T, Valachis A, et al. Discordance of PD-L1 status between primary and metastatic breast cancer: a systematic review and meta-analysis. Cancer Treat Rev. 2021; 99:102257.
Article25. Peg V, Lopez-Garcia MA, Comerma L, Peiro G, Garcia-Caballero T, Lopez AC, et al. PD-L1 testing based on the SP142 antibody in metastatic triple-negative breast cancer: summary of an expert round-table discussion. Future Oncol. 2021; 17:1209–18.26. Ghosh J, Chatterjee M, Ganguly S, Datta A, Biswas B, Mukherjee G, et al. PDL1 expression and its correlation with outcomes in non-metastatic triple-negative breast cancer (TNBC). Ecancermedicalscience. 2021; 15:1217.
Article27. Wang X, Liu Y. PD-L1 expression in tumor infiltrated lymphocytes predicts survival in triple-negative breast cancer. Pathol Res Pract. 2020; 216:152802.
Article28. Beckers RK, Selinger CI, Vilain R, Madore J, Wilmott JS, Harvey K, et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology. 2016; 69:25–34.
Article29. Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018; 19:40–50.
Article30. Gao G, Wang Z, Qu X, Zhang Z. Prognostic value of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: a systematic review and meta-analysis. BMC Cancer. 2020; 20:179.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concordance of Programmed DeathLigand 1 Expression between SP142 and 22C3/SP263 Assays in Triple-Negative Breast Cancer
- Programmed Death Ligand 1 Immunohistochemistry in TripleNegative Breast Cancer: Evaluation of Inter-Pathologist Concordance and Inter-Assay Variability
- Comparison of Programmed Cell Death Ligand 1 Status between Core Needle Biopsy and Surgical Specimens of Triple-Negative Breast Cancer
- Fatty acid synthetase expression in triple-negative breast cancer
- Correlation of PD-L1 Expression Tested by 22C3 and SP263 in Non-Small Cell Lung Cancer and Its Prognostic Effect on EGFR Mutation–Positive Lung Adenocarcinoma