Anat Cell Biol.
2024 Mar;57(1):129-142. 10.5115/acb.23.230.
Bisphosphonate’s effect on the tongue in adult male albino rats and the possible protective role of rutin: light and scanning electron microscopic study
- Affiliations
-
- 1Department of Histology and Cell Biology, Faculty of Medicine, Menoufia University, Al Menoufia, Egypt
- KMID: 2554247
- DOI: http://doi.org/10.5115/acb.23.230
Abstract
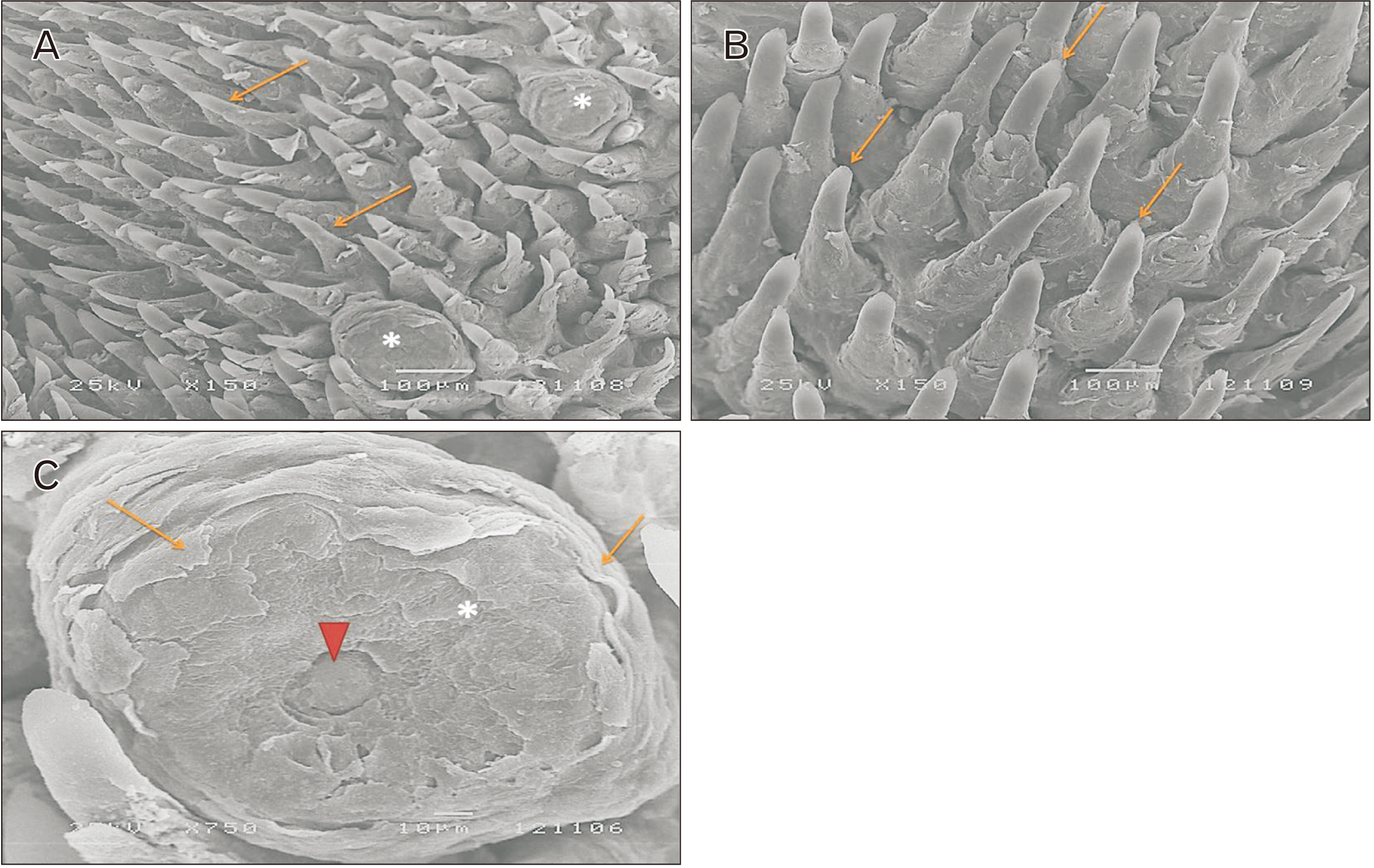
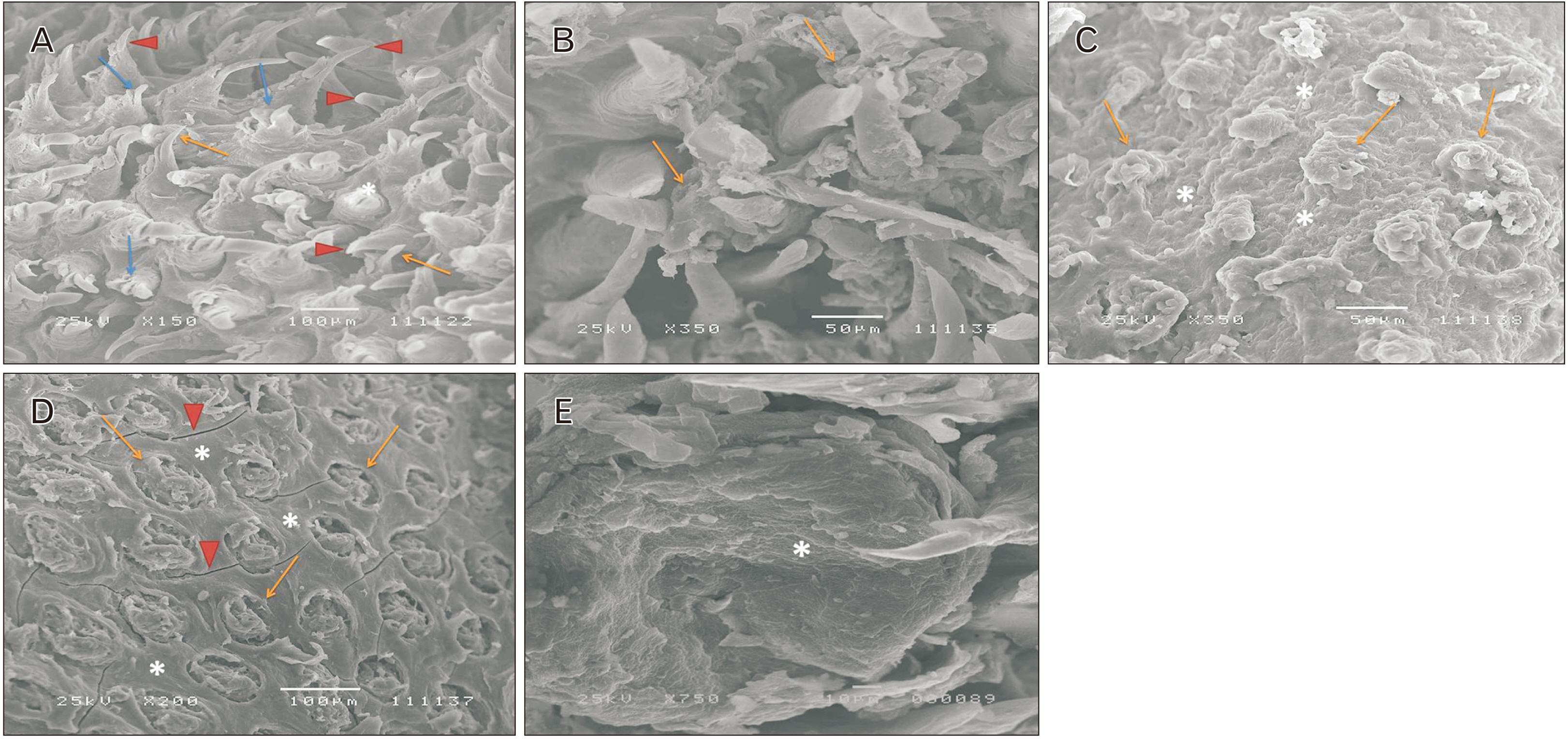
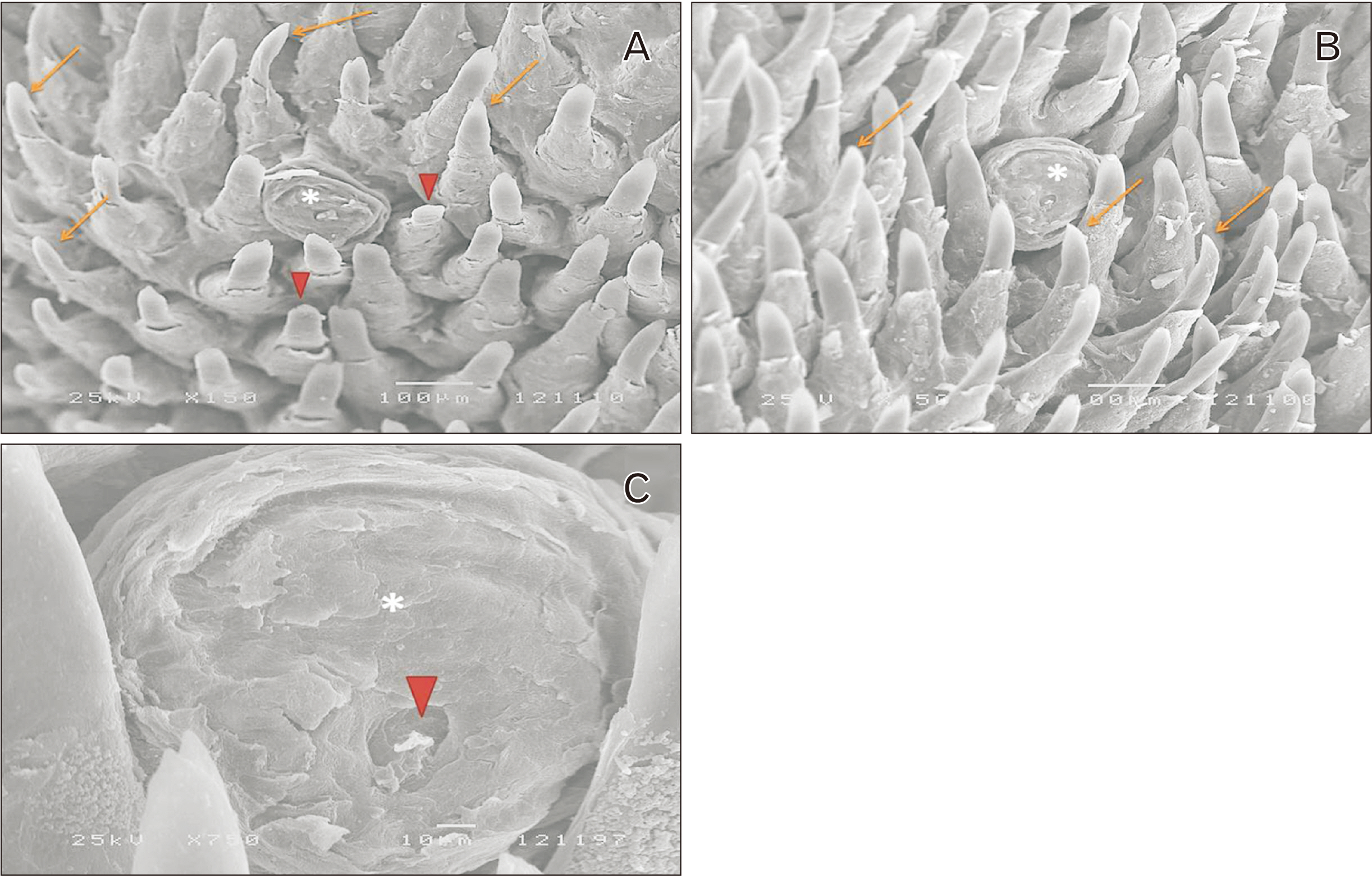
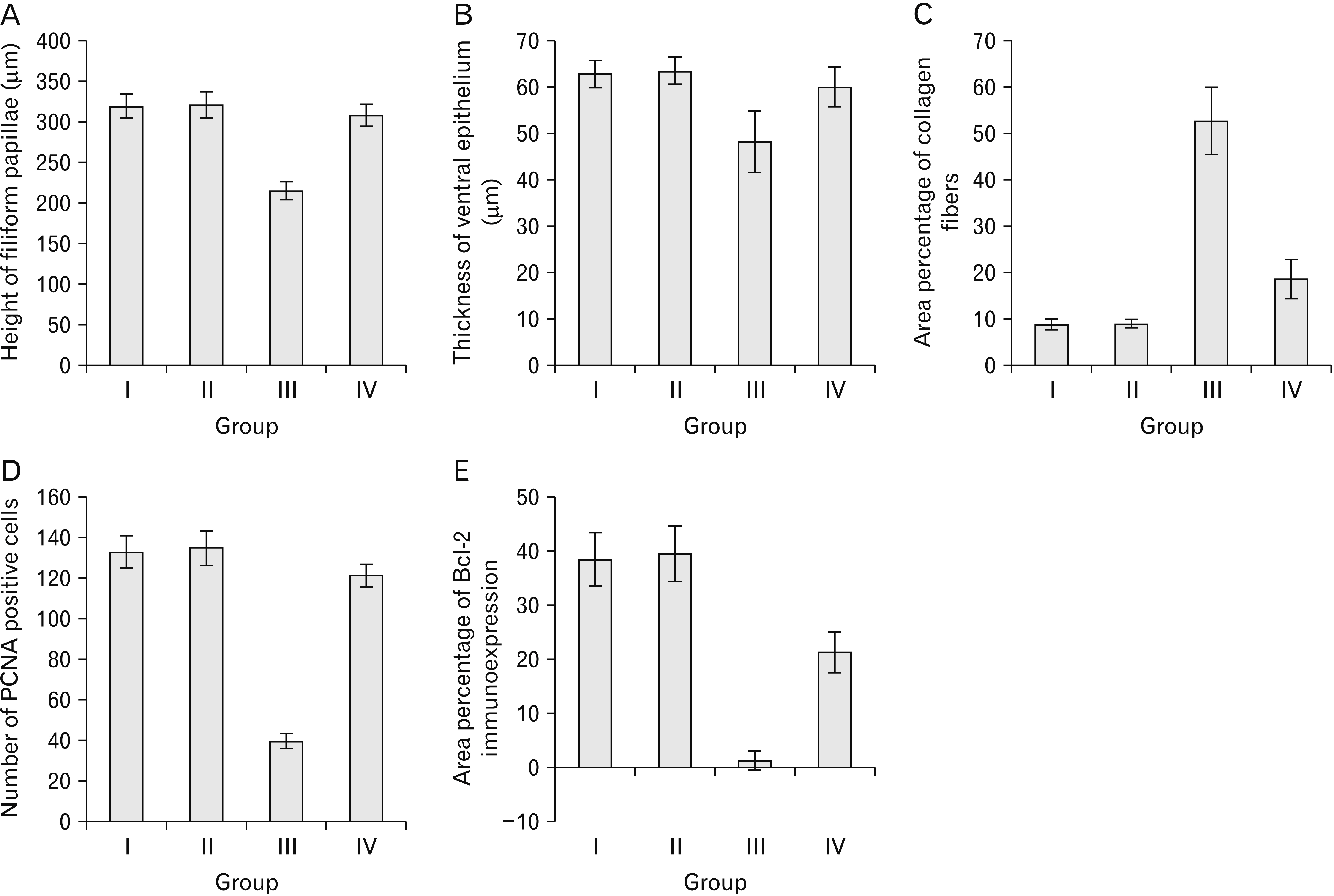
- Alendronate sodium (ALS) is a nitrogen-containing bisphosphonate used for the treatment of different bone disorders. However, its adverse effect on oral soft tissue has been detected. Rutin (RUT) is natural flavonoid with antioxidant and anti-inflammatory properties. This work aimed to investigate the possible effect of ALS on the tongue of adult male albino rats and to evaluate the possible protective role of RUT. Forty adult male albino rats were equally divided into four groups: group I (control), group II (RUT): Received RUT 50 mg/kg, group III (ALS): Received ALS 1 mg/kg, group IV (ALS+RUT): Received ALS and RUT with the same doses as pervious groups. The drugs were given once daily for 5 weeks. Tongue specimens were taken and processed for light and scanning electron microscopic inspection. ALS treated group revealed structural changes in the tongue in the form of decrease in the height of the filiform papillae with blunt ends, marked atrophy in some papillae with areas of focal loss, loss of some epithelial cells, pyknotic nuclei and cytoplasmic vacuoles in some epithelial cells. The lamina propria showed inflammatory cellular infiltration with congested blood vessels. Statistically, there were highly significant decrease in the number of proliferating cell nuclear antigen immunopositive cells, area percentage of Bcl-2 immunoexpression and highly significant increase in the collagen content compared to control group. Administration of RUT with ALS minimizes these changes. RUT protected the rat tongue against the histological and immunohistochemical changes induced by ALS through its antioxidant and anti-inflammatory properties.
Keyword
Figure
Reference
-
References
1. Zandi M, Dehghan A, Talimkhani I, Rezaeian L, Mohammad Gholi Mezerji N. 2019; Histological evaluation of the healing process of autografted mandibular bone defects in rats under treatment with zoledronate. J Craniomaxillofac Surg. 47:1779–86. DOI: 10.1016/j.jcms.2019.08.003. PMID: 31635981.
Article2. Oyhanart SR, Escudero ND, Mandalunis PM. 2015; Effect of alendronate on the mandible and long bones: an experimental study in vivo. Pediatr Res. 78:618–25. DOI: 10.1038/pr.2015.163. PMID: 26331769.
Article3. Psimma C, Psimma Z, Willems HC, Klüter WJ, van der Maarel-Wierink CD. 2022; Oral bisphosphonates: adverse effects on the oral mucosa not related to the jaw bones. A scoping review. Gerodontology. 39:330–8. DOI: 10.1111/ger.12590. PMID: 34725854. PMCID: PMC9787882.
Article4. Farag DB, Mehanny SS. 2020; Histopathological alterations of the intrinsic tongue muscles following zoledronic acid treatment in a rat model. Dent Med Probl. 57:131–6. DOI: 10.17219/dmp/115368. PMID: 32463600.
Article5. Badae NM, Ghazala RA, Zaki EI, Abdel-Ghani SA. 2019; Evaluating the therapeutic effect of Diallyl disulfide compared to that of Alderonate on Glucocorticoids induced osteoporosis in rats: Biochemical and histomorphometric analysis. Bull Egypt Soc Physiol Sci. 39:129–42. DOI: 10.21608/besps.2019.6704.1011.
Article6. Tokmak Özşahin ET, Çam B, Dere F, Kürkçü M, Evrüke C, Soames R, Oğuz Ö. 2017; The effect of alendronate sodium on trabecular bone structure in an osteoporotic rat model. Turk J Phys Med Rehabil. 63:165–73. DOI: 10.5606/tftrd.2017.164. PMID: 31453446. PMCID: PMC6648129.
Article7. Papamitsou T, Morsi-Yeroyannis A, Papanastasiou A, Bakalopoulos N, Dietrich EM, Karachrysafi S, Toskas A, Mareti E, Morsi-Yeroyanni A, Sioga A. 2020; Bisphosphonate's effect on tongue mucosa: an experimental electron microscopy study. Medicina (Kaunas). 56:51. DOI: 10.3390/medicina56020051. PMID: 31991568. PMCID: PMC7073723.
Article8. Donetti E, Gualerzi A, Sardella A, Lodi G, Carrassi A, Sforza C. 2014; Alendronate impairs epithelial adhesion, differentiation and proliferation in human oral mucosa. Oral Dis. 20:466–72. DOI: 10.1111/odi.12154. PMID: 23837876.
Article9. Theodora P, Stella F, Angeliki P, Eva-Maria D, Dimitris K, Alexandros T, Sofia K, Antonia S. 2018; Effect of alendronic acid on buccal mucosa. J Dent Oral Health. 4:1–6.10. Erdogan E, Ilgaz Y, Gurgor PN, Oztas Y, Topal T, Oztas E. 2015; Rutin ameliorates methotrexate induced hepatic injury in rats. Acta Cir Bras. 30:778–84. DOI: 10.1590/S0102-865020150110000009. PMID: 26647798.
Article11. Al-Hamadawi HA, Al-Ankoshy AAM, Alqershi KA. 2022; Studying the protective and therapeutic role of Rutin on the histological structure of the liver and some physiological parameters in Rats treated with ciprofloxacin. BNIHS. 140:1113–22.12. Hussani MF, Sabour AN. 2022; Effect of rutin on the histological structure of the heart and some biochemical indicators of oxidative stress in male Albino rats. PJMHS. 16:653–7. DOI: 10.53350/pjmhs22167653.
Article13. Thandavamoorthy P, Balan R, Subramaniyan J, Arumugam M, John B, Krishnan G, Ramasamy E, Mani GK, Rajendran R, Thiruvengadam D. 2014; Alleviative role of rutin against 4-Nitroquinoline-1-oxide (4-NQO) provoked oral squamous cell carcinoma in experimental animal model. J Pharm Res. 8:899–906.14. Motamedshariaty VS, Amel Farzad S, Nassiri-Asl M, Hosseinzadeh H. 2014; Effects of rutin on acrylamide-induced neurotoxicity. Daru. 22:27. DOI: 10.1186/2008-2231-22-27. PMID: 24524427. PMCID: PMC3927829.
Article15. Gaertner D, Hallman T, Claire Hankenson F, Batchelder MA. Fish RE, Brown MJ, Danneman PJ, Karas AZ, editors. Anesthesia and analgesia for laboratory rodents. Anesthesia and Analgesia in Laboratory Animals. 2nd ed. Academic Press;2008. p. 239–97. DOI: 10.1016/B978-012373898-1.50014-0.
Article16. Kiernan JA. Histological and histochemical methods: theory and practice. 5th ed. Scion;2015. p. 238–310.17. Ramos-Vara JA, Kiupel M, Baszler T, Bliven L, Brodersen B, Chelack B, Czub S, Del Piero F, Dial S, Ehrhart EJ, Graham T, Manning L, Paulsen D, Valli VE, West K. 2008; Suggested guidelines for immunohistochemical techniques in veterinary diagnostic laboratories. J Vet Diagn Invest. 20:393–413. DOI: 10.1177/104063870802000401. PMID: 18599844.
Article18. Ozer H, Yenicesu G, Arici S, Cetin M, Tuncer E, Cetin A. 2012; Immunohistochemistry with apoptotic-antiapoptotic proteins (p53, p21, bax, bcl-2), c-kit, telomerase, and metallothionein as a diagnostic aid in benign, borderline, and malignant serous and mucinous ovarian tumors. Diagn Pathol. 7:124. DOI: 10.1186/1746-1596-7-124. PMID: 22995373. PMCID: PMC3523067.
Article19. Piroeva I, Atanassova-Vladimirova S, Dimowa L, Sbirkova H, Radoslavov G, Hristov P, Shivachev BL. 2013; A simple and rapid scanning electron microscope preparative technique for observation of biological samples: application on bacteria and DNA samples. Bulgarian Chem Commun. 45:510–5.20. White SE, Malley J, Carton L, Dawson B. Basic and clinical biostatistics. 5th ed. McGraw-Hill Companies;2020.21. Fideles LS, de Miranda JAL, Martins CDS, Barbosa MLL, Pimenta HB, Pimentel PVS, Teixeira CS, Scafuri MAS, Façanha SO, Barreto JEF, Carvalho PMM, Scafuri AG, Araújo JL, Rocha JA, Vieira IGP, Ricardo NMPS, da Silva Campelo M, Ribeiro MENP, de Castro Brito GA, Cerqueira GS. 2020; Role of Rutin in 5-fluorouracil-induced intestinal mucositis: prevention of histological damage and reduction of inflammation and oxidative stress. Molecules. 25:2786. DOI: 10.3390/molecules25122786. PMID: 32560278. PMCID: PMC7356626.
Article22. Kharazmi M, Sjöqvist K, Warfvinge G. 2012; Oral ulcers, a little known adverse effect of alendronate: review of the literature. J Oral Maxillofac Surg. 70:830–6. DOI: 10.1016/j.joms.2011.03.046. PMID: 21816532.
Article23. Carvalho NS, Silva MM, Silva RO, Nicolau LA, Araújo TS, Costa DS, Sousa NA, Souza LK, Soares PM, Medeiros JV. 2016; Protective effects of simvastatin against alendronate-induced gastric mucosal injury in rats. Dig Dis Sci. 61:400–9. DOI: 10.1007/s10620-015-3890-7. PMID: 26403426.
Article24. Kassab AA. 2019; Effect of Fosamax on the duodenal mucosa in adult male albino rats and the possible protection by nigella sativa oil: a histological and immunohistochemical study. Egypt J Histol. 42:900–14. DOI: 10.21608/ejh.2019.10902.1105.
Article25. Kassab AA, Moustafa KA, Abd-El-Hafez AA. 2020; The possible protective role of pumpkin seed oil in ameliorating tongue mucosal damage induced by orlistat in adult male albino rats: a light and scanning electron microscopic study. Egypt J Histol. 43:975–87. DOI: 10.21608/ejh.2020.25027.1258.
Article26. Pourgonabadi S, Ghorbani A, Tayarani Najarn Z, Mousavi SH. 2018; In vitro assessment of alendronate toxic and apoptotic effects on human dental pulp stem cells. Iran J Basic Med Sci. 21:905–10. DOI: 10.22038/IJBMS.2018.22877.5816. PMID: 30524690. PMCID: PMC6272077.27. Saso L, Suzen S, Borges F, Csont T. 2020; Chemistry and pharmacology of modulators of oxidative stress. Curr Med Chem. 27:2038–9. DOI: 10.2174/092986732713200425202016. PMID: 32368965.
Article28. Thoma A, Lightfoot AP. 2018; NF-kB and inflammatory cytokine signalling: role in skeletal muscle atrophy. Adv Exp Med Biol. 1088:267–79. DOI: 10.1007/978-981-13-1435-3_12. PMID: 30390256.
Article29. Tian C, Liu X, Chang Y, Wang R, Yang M, Liu M. 2021; Rutin prevents inflammation induced by lipopolysaccharide in RAW 264.7 cells via conquering the TLR4-MyD88-TRAF6-NF-κB signalling pathway. J Pharm Pharmacol. 73:110–7. DOI: 10.1093/jpp/rgaa015. PMID: 33791807.
Article30. Li Y, Qin L, Ying L, Dong H, Wang D. 2019; Rutin prevents retinal ganglion cell death and exerts protective effects by regulating transforming growth factor-β2/Smad2/3Akt/PTEN signaling in experimental rat glaucoma. Trop J Pharm Res. 18:985–93. DOI: 10.4314/tjpr.v18i5.11.
Article31. Bai L, Li A, Gong C, Ning X, Wang Z. 2020; Protective effect of rutin against bleomycin induced lung fibrosis: Involvement of TGF-β1/α-SMA/Col I and III pathway. Biofactors. 46:637–44. DOI: 10.1002/biof.1629. PMID: 32233122.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Studies on intestinal trematodes in Korea XIX. Light and scanning electron microscopy of Fibricola seoulensis collected from albino rats treated with praziquantel
- Scanning electron microscopic study of capillary change in bleomycin-induced pulmonary fibrosis
- Does oral ciprofloxacin affect the structure of thoracic aorta in adult and senile male albino rats? A clue to fluoroquinolones-induced risk of aortic dissection
- Morphological, ultrastructural, and biochemical changes induced by sodium fluoride in the tongue of adult male albino rat and the ameliorative effect of resveratrol
- Morpholgical Study of Korean Pubic Louse , Phthirus pubis ( Linnaeus , 1758 ) by Light and Scanning Electron Microscopy