J Korean Med Sci.
2024 Apr;39(12):e116. 10.3346/jkms.2024.39.e116.
Government Initiatives for Research Ethics During COVID-19 Pandemic in Korea
- Affiliations
-
- 1Department of the History of Medicine and Medical Humanities, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2554210
- DOI: http://doi.org/10.3346/jkms.2024.39.e116
Abstract
- Background
Despite the growing necessity for government-led policy changes on clinical research ethics during pandemic, the scope of previous literature is limited to Korean government’s pandemic response strategies or reflections of research ethics at the level of institutions and academic societies. This paper examines the proactive policy changes and responses by the South Korean government in addressing the challenges and issues of research ethics against the backdrop of the urgency of rapid development and emergency supply of medical products during the coronavirus disease 2019 (COVID-19) pandemic.
Methods
We conducted searches of various government documents, using predetermined keywords related to research ethics and integrity during the COVID-19 pandemic. Only documents issued by governments or public institutions were included. A total of 24 documents were selected for analysis. They were divided into two phases: the first phase for urgent response (January 2020–February 2021) and the second phase (March 2021–February 2023) for long-term preparedness.
Results
The Korean government recommended several measures of research governance to accelerate the ethical review of COVID-related research to be shortened less than one week: the joint operation of Institutional Review Boards (IRBs), exempted or expedited review by a special review committee, guidelines for urgent reviews, and designation of the Korean Academy of Medical Sciences as the supervising agency for the Clinical Trial Safety Support Institution as well as the Central IRB. It allowed temporary non-face-to-face methods for informed consent process (telephone explanations and a photo of the original signed consent) and clinical trials (telephone counselling and prescription, proxy prescription, and drug delivery and supply to clinical trial participants, and online ethics training).
Conclusion
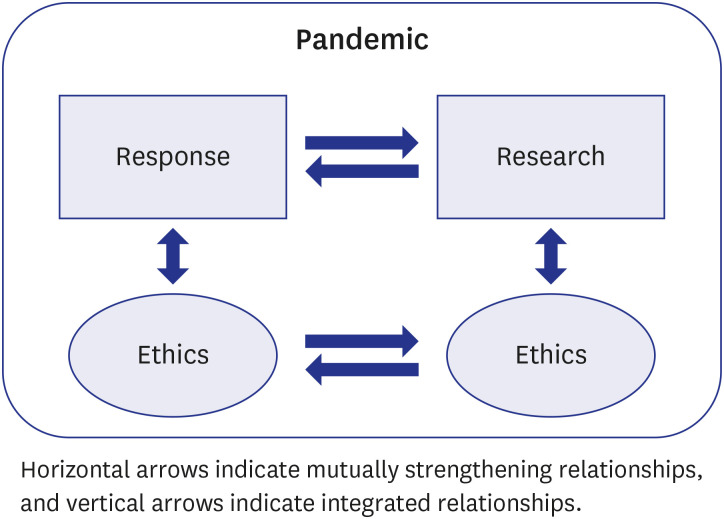
As a result of South Korea’s commitment to ethical principles in their pandemic response, the medical system did not experience collapses due to the pandemic, and pandemic research was conducted with careful ethical considerations. The pandemic ethics immunization during the Middle East respiratory syndrome epidemic in 2015 laid the foundation for prompt government initiatives that ensured both pandemic research ethics and pandemic response ethics.
Figure
Reference
-
1. Solbakk JH, Bentzen HB, Holm S, Heggestad AK, Hofmann B, Robertsen A, et al. Back to WHAT? The role of research ethics in pandemic times. Med Health Care Philos. 2021; 24(1):3–20. PMID: 33141289.2. Park S, Lim HJ, Park J, Choe YH. Impact of COVID-19 pandemic on biomedical publications and their citation frequency. J Korean Med Sci. 2022; 37(40):e296. PMID: 36254532.3. Faust A, Sierawska A, Krüger K, Wisgalla A, Hasford J, Strech D. Challenges and proposed solutions in making clinical research on COVID-19 ethical: a status quo analysis across German research ethics committees. BMC Med Ethics. 2021; 22(1):96. PMID: 34281535.4. Singh S, Cadigan RJ, Moodley K. Challenges to biobanking in LMICs during COVID-19: time to reconceptualise research ethics guidance for pandemics and public health emergencies? J Med Ethics. 2022; 48(7):466–471. PMID: 33980656.5. De Sutter E, Lalova-Spinks T, Borry P, Valcke P, Kindt E, Negrouk A, et al. Rethinking informed consent in the time of COVID-19: an exploratory survey. Front Med (Lausanne). 2022; 9:995688. PMID: 36237540.6. Barroga E, Matanguihan GJ. Fundamental shifts in research, ethics and peer review in the era of the COVID-19 pandemic. J Korean Med Sci. 2020; 35(45):e395. PMID: 33230986.7. Sisk BA, DuBois J. Research ethics during a pandemic: a call for normative and empirical analysis. Am J Bioeth. 2020; 20(7):82–84.8. Ten Have H. The COVID-19 Pandemic and Global Bioethics. Cham, Switzerland: Springer;2022.9. Zhang H, Shao F, Gu J, Li L, Wang Y. Ethics committee reviews of applications for research studies at 1 hospital in China during the 2019 novel coronavirus epidemic. JAMA. 2020; 323(18):1844–1846. PMID: 32202608.10. Rothwell E, Brassil D, Barton-Baxter M, Brownley KA, Dickert NW, Ford DE, et al. Informed consent: old and new challenges in the context of the COVID-19 pandemic. J Clin Transl Sci. 2021; 5(1):e105.11. Jones XM, Zimba O, Gupta L. Informed consent for scholarly articles during the COVID-19 pandemic. J Korean Med Sci. 2021; 36(3):e31. PMID: 33463097.12. Tosoni S, Voruganti I, Lajkosz K, Mustafa S, Phillips A, Kim SJ, et al. Patient consent preferences on sharing personal health information during the COVID-19 pandemic: “the more informed we are, the more likely we are to help”. BMC Med Ethics. 2022; 23(1):53. PMID: 35596210.13. Voit K, Skuban-Eiseler T, Orzechowski M, Steger F. Informed consent in COVID-19-research: an ethical analysis of clinical studies performed during the pandemic. Healthcare (Basel). 2023; 11(12):1793. PMID: 37372911.14. Ma X, Wang Y, Gao T, He Q, He Y, Yue R, et al. Challenges and strategies to research ethics in conducting COVID-19 research. J Evid Based Med. 2020; 13(2):173–177. PMID: 32445288.15. Gaur PS, Zimba O, Agarwal V, Gupta L. Reporting survey based studies - a primer for authors. J Korean Med Sci. 2020; 35(45):e398. PMID: 33230988.16. Zimba O, Gasparyan AY. Designing, conducting, and reporting survey studies: a primer for researchers. J Korean Med Sci. 2023; 38(48):e403. PMID: 38084027.17. Yang JH. Korean Good Clinical Practice. J Korean Soc Clin Pharmacol Ther. 1996; 4(1):35–48.18. Mehta P, Zimba O, Gasparyan AY, Seiil B, Yessirkepov M. Ethics committees: structure, roles, and issues. J Korean Med Sci. 2023; 38(25):e198. PMID: 37365729.19. Kim OJ, Park BJ, Sohn DR, Lee SM, Shin SG. Current status of the institutional review boards in Korea: constitution, operation, and policy for protection of human research participants. J Korean Med Sci. 2003; 18(1):3–10. PMID: 12589079.20. Kim OJ. Bioethics and research ethics in Korea after Hwang Woo-Suk Scandal. The National Academy of Medicine of Korea. The Hwang Woo-Suk Scandal 10 Years Ago From the Present Perspective. Seoul, Korea: Park Young-Sa;2018. p. 37–49.21. Lee MJ, Kim OJ, Cho EH, Jeon JH, Lee DH, Lee S, et al. Introduction of the National R&D Innovation Act and research ethics in Korea. Asia Pac J Health Law Ethics. 2021; 14(2):155–192.22. Dighe A, Cattarino L, Cuomo-Dannenburg G, Skarp J, Imai N, Bhatia S, et al. Response to COVID-19 in South Korea and implications for lifting stringent interventions. BMC Med. 2020; 18(1):321. PMID: 33032601.23. Issac A, Stephen S, Jacob J, Vr V, Radhakrishnan RV, Krishnan N, et al. The pandemic league of COVID-19: Korea versus the United States, with lessons for the entire world. J Prev Med Public Health. 2020; 53(4):228–232. PMID: 32752591.24. Jeong E, Hagose M, Jung H, Ki M, Flahault A. Understanding South Korea’s response to the COVID-19 outbreak: a real-time analysis. Int J Environ Res Public Health. 2020; 17(24):9571. PMID: 33371309.25. Kim SW. COVID-19 outbreak in Daegu City, Korea and response to COVID-19: how have we dealt and what are the lessons? J Korean Med Sci. 2022; 37(50):e356. PMID: 36573388.26. Park K, Baek HJ. Contextual response to the COVID-19 pandemic from the experience of South Korea. Public Health. 2023; 222:e7–e8. PMID: 36045020.27. Lee D, Heo K, Seo Y, Ahn H, Jung K, Lee S, et al. Flattening the curve on COVID-19: South Korea’s measures in tackling initial outbreak of coronavirus. Am J Epidemiol. 2021; 190(4):496–505. PMID: 33106843.28. Yang TU, Noh JY, Song JY, Cheong HJ, Kim WJ. How lessons learned from the 2015 Middle East respiratory syndrome outbreak affected the response to coronavirus disease 2019 in the Republic of Korea. Korean J Intern Med. 2021; 36(2):271–285. PMID: 32872738.29. Park J, Chung E. Learning from past pandemic governance: early response and public-private partnerships in testing of COVID-19 in South Korea. World Dev. 2021; 137:105198. PMID: 32982017.30. Lee SI. Costly lessons from the 2015 Middle East respiratory syndrome coronavirus outbreak in Korea. J Prev Med Public Health. 2015; 48(6):274–276. PMID: 26639740.31. Kim OJ. Ethical perspectives on the Middle East respiratory syndrome coronavirus epidemic in Korea. J Prev Med Public Health. 2016; 49(1):18–22. PMID: 26841881.32. Wu F, Hu Q, Zhu C, Wang H, Yu Q, Sun H. New structural economic analysis of anti-COVID-19 pandemic model of BEST region. Int J Environ Res Public Health. 2021; 18(15):7822. PMID: 34360114.33. Lee S, Wong R. COVID-19 responses of South Korea as hybrids of governance modes. Front Public Health. 2021; 9:654945. PMID: 34604149.34. Jeon J, Kim H, Yu KS. The impact of COVID-19 on conduct of clinical trials for medical products in Korea. J Korean Med Sci. 2020; 35(36):e329. PMID: 32924344.35. Yoo SH, Sim JA, Shin J, Keam B, Park JB, Shin A. The impact of COVID-19 on cancer care in a tertiary hospital in Korea: possible collateral damage to emergency care. Epidemiol Health. 2022; 44:e2022044. PMID: 35538696.36. Moon MJ. Fighting COVID-19 with agility, transparency, and participation: wicked policy problems and new governance challenges. Public Adm Rev. 2020; 80(4):651–656. PMID: 32836434.37. Oh J, Lee JK, Schwarz D, Ratcliffe HL, Markuns JF, Hirschhorn LR. National response to COVID-19 in the Republic of Korea and lessons learned for other countries. Health Syst Reform. 2020; 6(1):e1753464. PMID: 32347772.38. Choi JY, Lee SH, Jamal T. Smart Korea: governance for smart justice during a global pandemic. J Sustain Tour. 2021; 29(2-3):541–550.39. Lee S. Steering the private sector in COVID-19 diagnostic test kit development in South Korea. Front Public Health. 2020; 8:563525. PMID: 33282810.40. Kang C, Lee I. COVID-19 pandemic, transparency, and “Polidemic” in the Republic of Korea. Asian Bioeth Rev. 2021; 13(2):213–224. PMID: 33727965.41. You K, Kim D, Kim OJ. Application and expansion of the harm principle to the restrictions of liberty in the COVID-19 public health crisis: focusing on the revised bill of the March 2020 「Infectious Disease Control and Prevention Act」. Korean Soc Law Med. 2020; 21(2):105–162.42. Fang Y, Kim OJ. The ethics of COVID-19 vaccine allocation. Korean J Med Ethics. 2021; 24(2):121–137.43. You K, Kim OJ. A review of the justifications for mandatory vaccination policies in response to the COVID-19 public health crisis. Korean J Med Ethics. 2022; 25(1):1–20.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fundamental Shifts in Research, Ethics and Peer Review in the Era of the COVID-19 Pandemic
- COVID-19 and abortion right
- The Management of Thyroid Disease in COVID-19 Pandemic
- Assessment and Management of Dysphagia during the COVID-19 Pandemic
- Opinion on the practice of cremation funeral for patients who died of COVID-19