Korean J Physiol Pharmacol.
2024 Mar;28(2):165-181. 10.4196/kjpp.2024.28.2.165.
Somatodendritic organization of pacemaker activity in midbrain dopamine neurons
- Affiliations
-
- 1Department of Physiology, Sungkyunkwan University School of Medicine, Suwon 16419, Korea
- KMID: 2553528
- DOI: http://doi.org/10.4196/kjpp.2024.28.2.165
Abstract
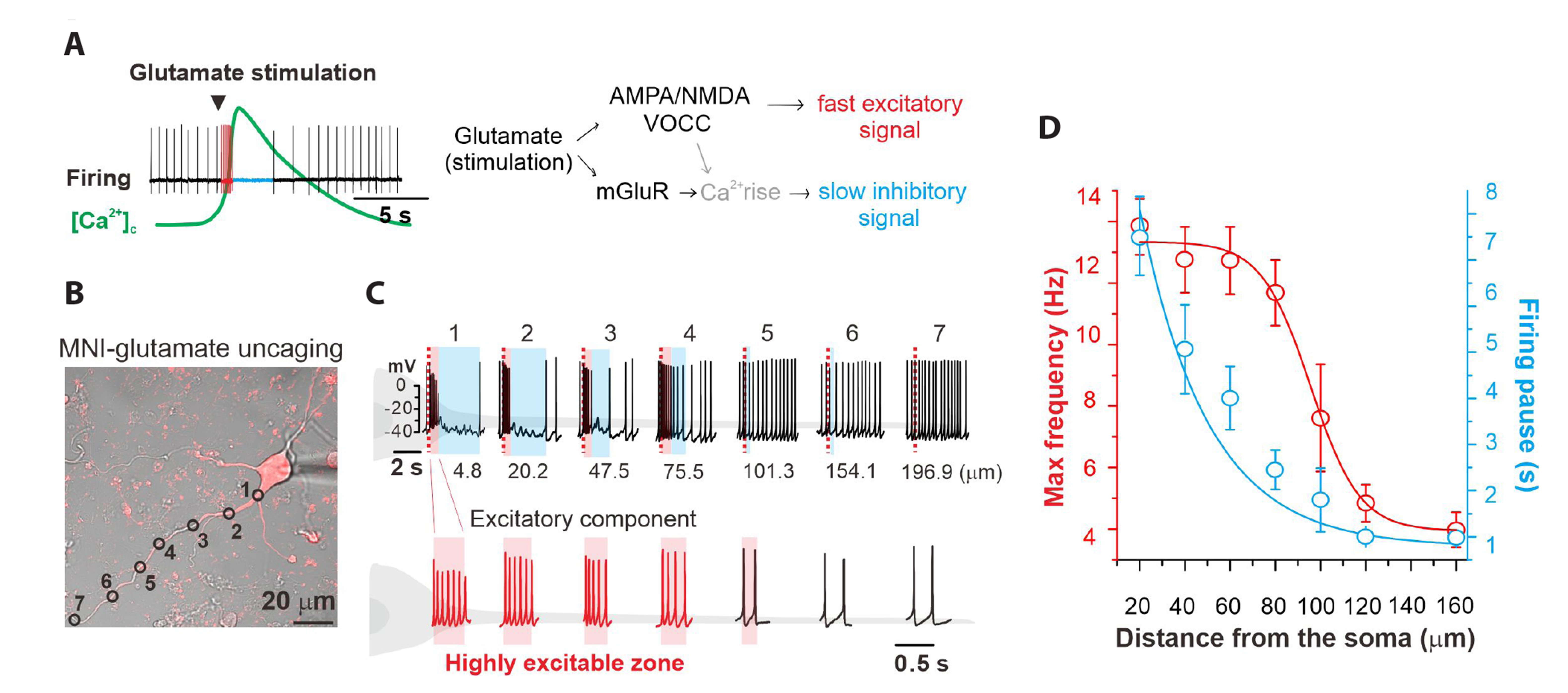
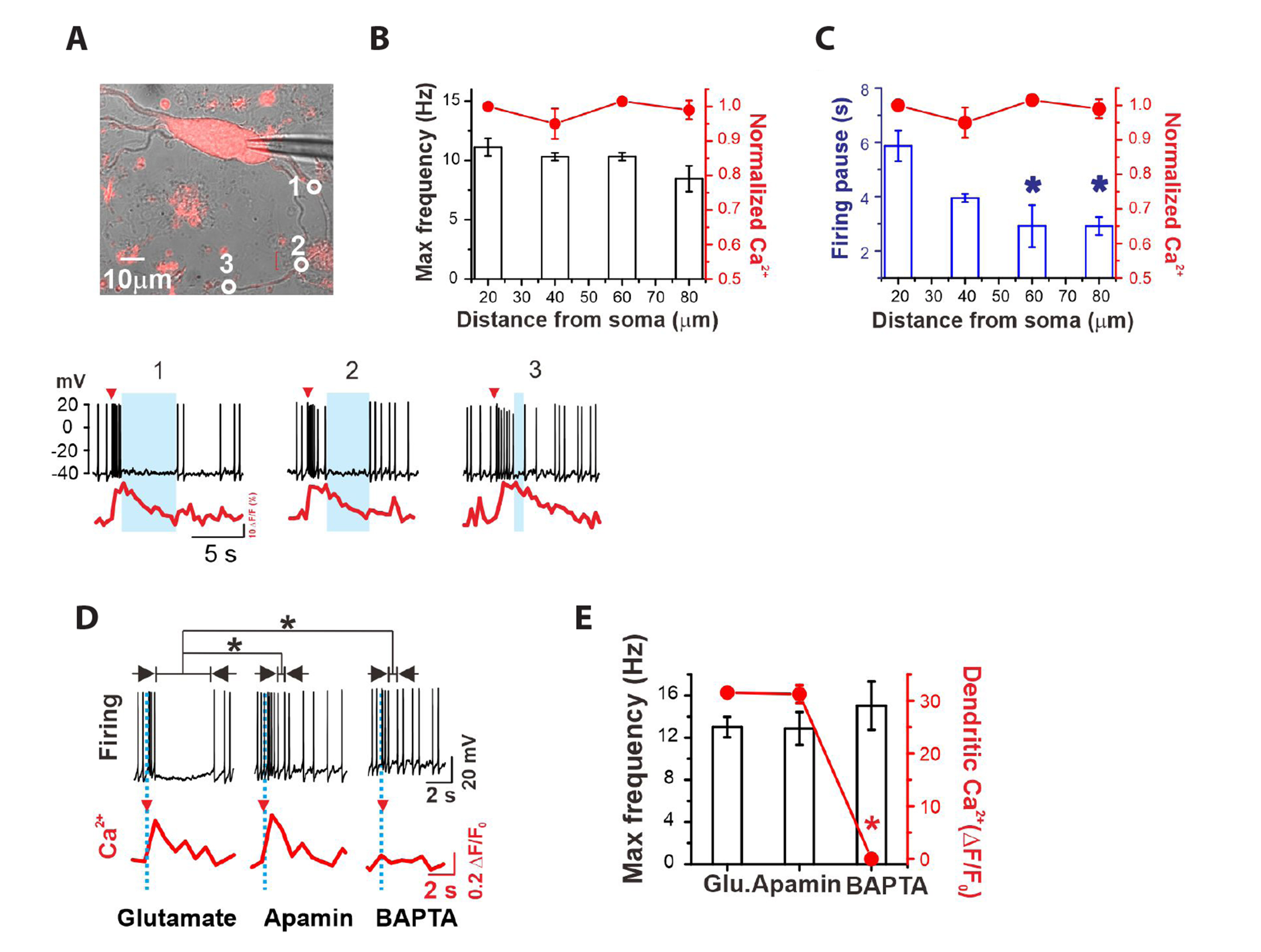
- The slow and regular pacemaking activity of midbrain dopamine (DA) neurons requires proper spatial organization of the excitable elements between the soma and dendritic compartments, but the somatodendritic organization is not clear. Here, we show that the dynamic interaction between the soma and multiple proximal dendritic compartments (PDCs) generates the slow pacemaking activity in DA neurons. In multipolar DA neurons, spontaneous action potentials (sAPs) consistently originate from the axon-bearing dendrite. However, when the axon initial segment was disabled, sAPs emerge randomly from various primary PDCs, indicating that multiple PDCs drive pacemaking. Ca2+ measurements and local stimulation/perturbation experiments suggest that the soma serves as a stably-oscillating inertial compartment, while multiple PDCs exhibit stochastic fluctuations and high excitability. Despite the stochastic and excitable nature of PDCs, their activities are balanced by the large centrally-connected inertial soma, resulting in the slow synchronized pacemaking rhythm. Furthermore, our electrophysiological experiments indicate that the soma and PDCs, with distinct characteristics, play different roles in glutamate-induced burst-pause firing patterns. Excitable PDCs mediate excitatory burst responses to glutamate, while the large inertial soma determines inhibitory pause responses to glutamate. Therefore, we could conclude that this somatodendritic organization serves as a common foundation for both pacemaker activity and evoked firing patterns in midbrain DA neurons.
Keyword
Figure
Cited by 2 articles
-
Haloperidol, a typical antipsychotic, inhibits 5-HT3 receptor-mediated currents in NCB-20 cells: a whole-cell patch-clamp study
Yong Soo Park, Gyu Min Kim, Ho Jun Sung, Ju Yeong Yu, Ki-Wug Sung
Korean J Physiol Pharmacol. 2025;29(3):349-358. doi: 10.4196/kjpp.24.320.Quetiapine competitively inhibits 5-HT3 receptor-mediated currents in NCB20 neuroblastoma cells
Yong Soo Park, Gyu Min Kim, Ho Jun Sung, Ju Yeong Yu, Ki-Wug Sung
Korean J Physiol Pharmacol. 2025;29(3):373-384. doi: 10.4196/kjpp.24.363.
Reference
-
1. Magee JC. 2000; Dendritic integration of excitatory synaptic input. Nat Rev Neurosci. 1:181–190. DOI: 10.1038/35044552. PMID: 11257906.2. Stuart GJ, Spruston N. 2015; Dendritic integration: 60 years of progress. Nat Neurosci. 18:1713–1721. DOI: 10.1038/nn.4157. PMID: 26605882.
Article3. Grace AA, Bunney BS. 1983; Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 10:301–315. DOI: 10.1016/0306-4522(83)90135-5. PMID: 6633863.
Article4. Grace AA, Bunney BS. 1983; Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--2. Action potential generating mechanisms and morphological correlates. Neuroscience. 10:317–331. DOI: 10.1016/0306-4522(83)90136-7. PMID: 6633864.
Article5. Grace AA, Onn SP. 1989; Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 9:3463–3481. DOI: 10.1523/JNEUROSCI.09-10-03463.1989. PMID: 2795134. PMCID: PMC6569889.
Article6. Cossette M, Lecomte F, Parent A. 2005; Morphology and distribution of dopaminergic neurons intrinsic to the human striatum. J Chem Neuroanat. 29:1–11. DOI: 10.1016/j.jchemneu.2004.08.007. PMID: 15589697.
Article7. Häusser M, Stuart G, Racca C, Sakmann B. 1995; Axonal initiation and active dendritic propagation of action potentials in substantia nigra neurons. Neuron. 15:637–647. DOI: 10.1016/0896-6273(95)90152-3. PMID: 7546743.
Article8. Gentet LJ, Williams SR. 2007; Dopamine gates action potential backpropagation in midbrain dopaminergic neurons. J Neurosci. 27:1892–1901. DOI: 10.1523/JNEUROSCI.5234-06.2007. PMID: 17314285. PMCID: PMC6673536.
Article9. Wilson CJ, Callaway JC. 2000; Coupled oscillator model of the dopaminergic neuron of the substantia nigra. J Neurophysiol. 83:3084–3100. DOI: 10.1152/jn.2000.83.5.3084. PMID: 10805703.
Article10. Kang Y, Kitai ST. 1993; Calcium spike underlying rhythmic firing in dopaminergic neurons of the rat substantia nigra. Neurosci Res. 18:195–207. DOI: 10.1016/0168-0102(93)90055-U. PMID: 7907413.
Article11. Guzman JN, Sánchez-Padilla J, Chan CS, Surmeier DJ. 2009; Robust pacemaking in substantia nigra dopaminergic neurons. J Neurosci. 29:11011–11019. DOI: 10.1523/JNEUROSCI.2519-09.2009. PMID: 19726659. PMCID: PMC2784968.
Article12. Blythe SN, Wokosin D, Atherton JF, Bevan MD. 2009; Cellular mechanisms underlying burst firing in substantia nigra dopamine neurons. J Neurosci. 29:15531–15541. DOI: 10.1523/JNEUROSCI.2961-09.2009. PMID: 20007477. PMCID: PMC2834564.
Article13. González-Cabrera C, Meza R, Ulloa L, Merino-Sepúlveda P, Luco V, Sanhueza A, Oñate-Ponce A, Bolam JP, Henny P. 2017; Characterization of the axon initial segment of mice substantia nigra dopaminergic neurons. J Comp Neurol. 525:3529–3542. DOI: 10.1002/cne.24288. PMID: 28734032.
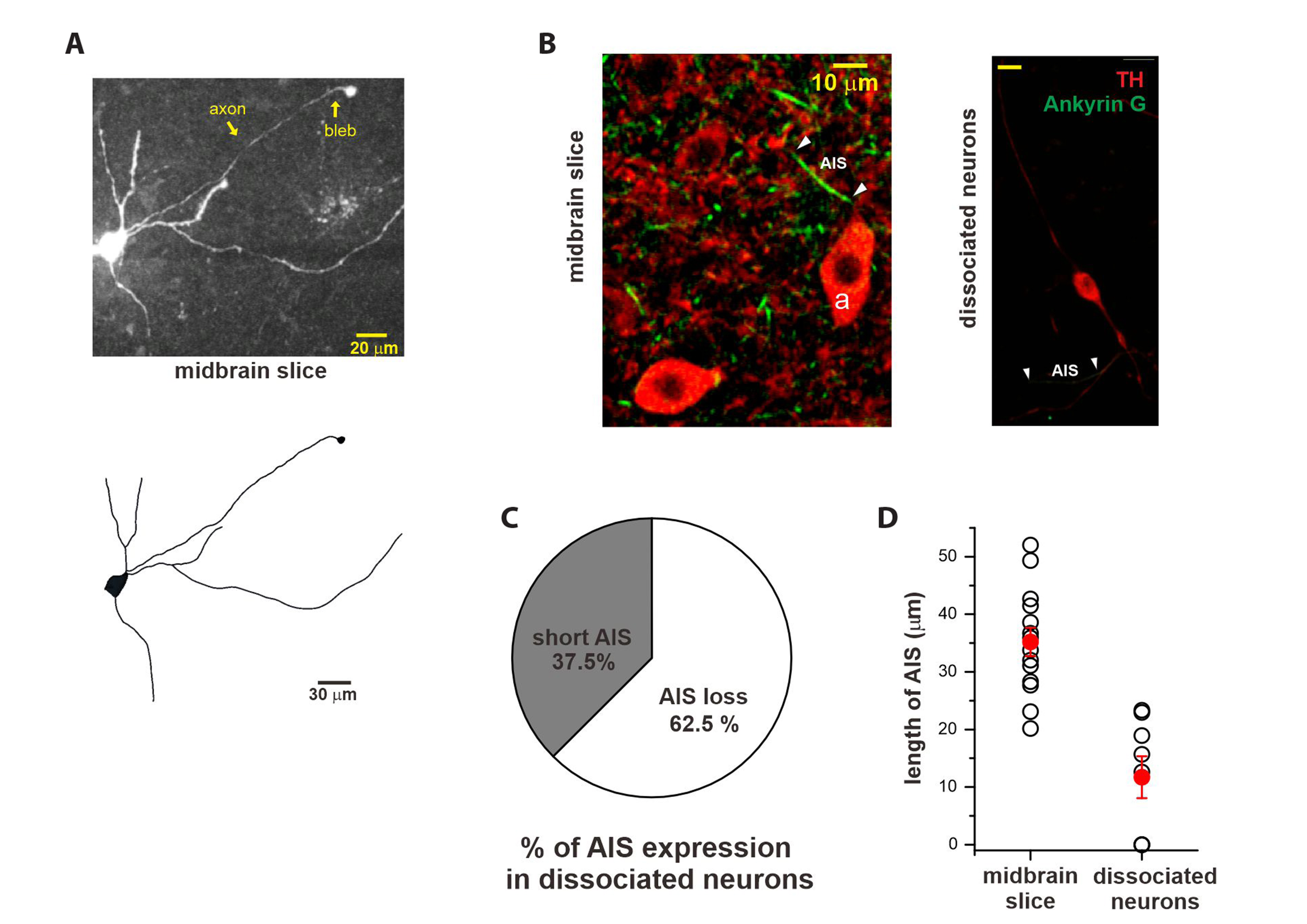
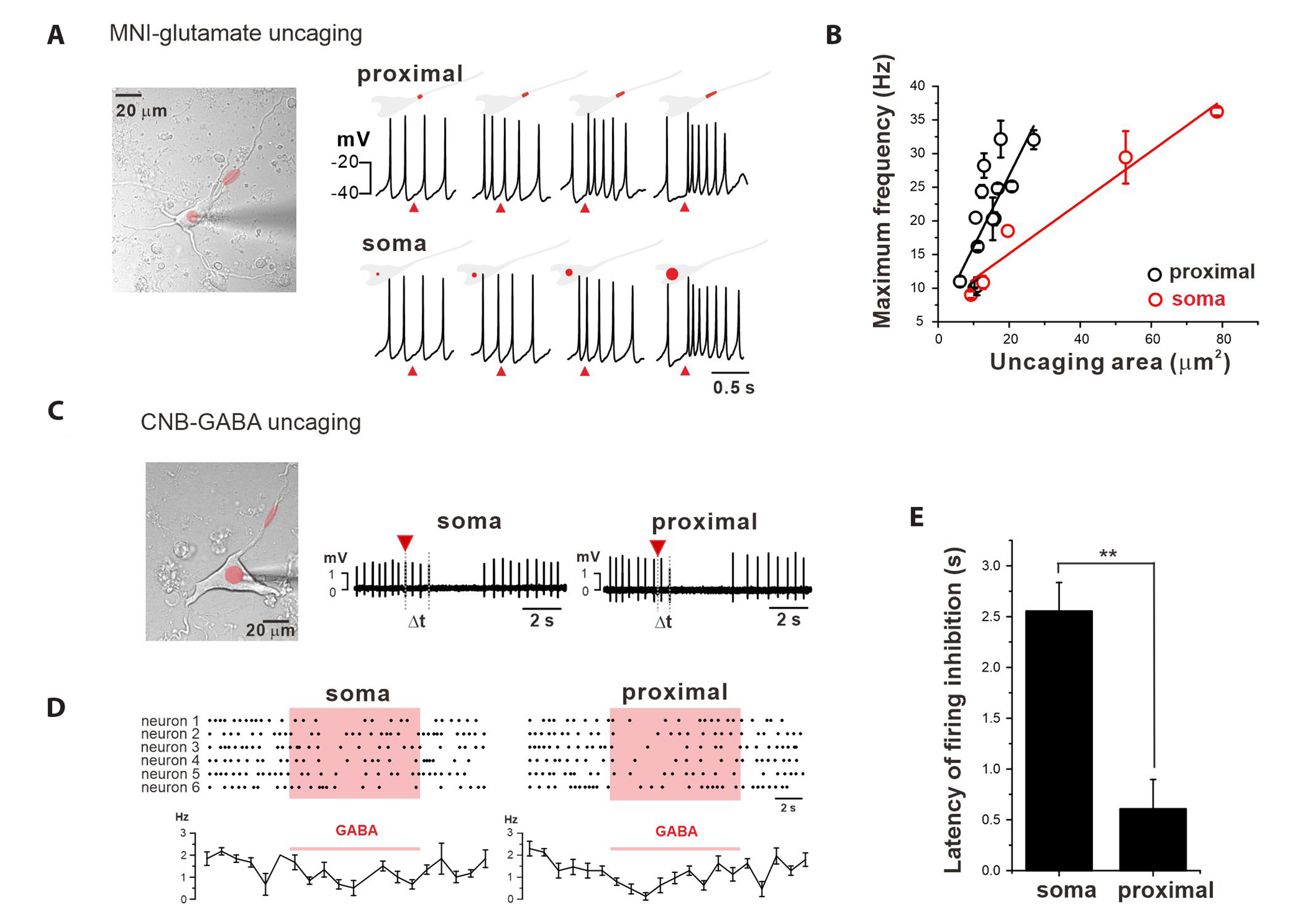
Article14. Jang J, Um KB, Jang M, Kim SH, Cho H, Chung S, Kim HJ, Park MK. 2014; Balance between the proximal dendritic compartment and the soma determines spontaneous firing rate in midbrain dopamine neurons. J Physiol. 592:2829–2844. DOI: 10.1113/jphysiol.2014.275032. PMID: 24756642. PMCID: PMC4221823.
Article15. Medvedev GS, Wilson CJ, Callaway JC, Kopell N. 2003; Dendritic synchrony and transient dynamics in a coupled oscillator model of the dopaminergic neuron. J Comput Neurosci. 15:53–69. DOI: 10.1023/A:1024422802673. PMID: 12843695.16. Grace AA, Floresco SB, Goto Y, Lodge DJ. 2007; Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 30:220–227. DOI: 10.1016/j.tins.2007.03.003. PMID: 17400299.
Article17. Pucak ML, Grace AA. 1994; Regulation of substantia nigra dopamine neurons. Crit Rev Neurobiol. 9:67–89.18. Overton PG, Clark D. 1997; Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 25:312–334. DOI: 10.1016/S0165-0173(97)00039-8. PMID: 9495561.
Article19. Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. 2002; Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 114:475–492. DOI: 10.1016/S0306-4522(02)00267-1. PMID: 12204216.
Article20. Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. 2009; Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 324:1080–1084. DOI: 10.1126/science.1168878. PMID: 19389999. PMCID: PMC5262197.
Article21. Wise RA. 2009; Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 32:517–524. DOI: 10.1016/j.tins.2009.06.004. PMID: 19758714. PMCID: PMC2755633.
Article22. Dawson TM, Dawson VL. 2003; Molecular pathways of neurodegeneration in Parkinsonʼs disease. Science. 302:819–822. DOI: 10.1126/science.1087753. PMID: 14593166.
Article23. Surmeier DJ, Guzman JN, Sanchez J, Schumacker PT. 2012; Physiological phenotype and vulnerability in Parkinsonʼs disease. Cold Spring Harb Perspect Med. 2:a009290. DOI: 10.1101/cshperspect.a009290. PMID: 22762023. PMCID: PMC3385938.
Article24. Grace AA. 1988; In vivo and in vitro intracellular recordings from rat midbrain dopamine neurons. Ann N Y Acad Sci. 537:51–76. DOI: 10.1111/j.1749-6632.1988.tb42096.x. PMID: 2849358.
Article25. Um KB, Hahn S, Kim SW, Lee YJ, Birnbaumer L, Kim HJ, Park MK. 2021; TRPC3 and NALCN channels drive pacemaking in substantia nigra dopaminergic neurons. Elife. 10:e70920. DOI: 10.7554/eLife.70920. PMID: 34409942. PMCID: PMC8456572.
Article26. Hahn S, Um KB, Kim SW, Kim HJ, Park MK. 2023; Proximal dendritic localization of NALCN channels underlies tonic and burst firing in nigral dopaminergic neurons. J Physiol. 601:171–193. DOI: 10.1113/JP283716. PMID: 36398712.
Article27. Jang M, Um KB, Jang J, Kim HJ, Cho H, Chung S, Park MK. 2015; Coexistence of glutamatergic spine synapses and shaft synapses in substantia nigra dopamine neurons. Sci Rep. 5:14773. DOI: 10.1038/srep14773. PMID: 26435058. PMCID: PMC4593176.
Article28. Choi YM, Kim SH, Uhm DY, Park MK. 2003; Glutamate-mediated [Ca2+]c dynamics in spontaneously firing dopamine neurons of the rat substantia nigra pars compacta. J Cell Sci. 116:2665–2675. DOI: 10.1242/jcs.00481. PMID: 12746490.
Article29. Hahn J, Tse TE, Levitan ES. 2003; Long-term K+ channel-mediated dampening of dopamine neuron excitability by the antipsychotic drug haloperidol. J Neurosci. 23:10859–10866. DOI: 10.1523/JNEUROSCI.23-34-10859.2003. PMID: 14645479. PMCID: PMC6740975.
Article30. Ping HX, Shepard PD. 1999; Blockade of SK-type Ca2+-activated K+ channels uncovers a Ca2+-dependent slow afterdepolarization in nigral dopamine neurons. J Neurophysiol. 81:977–984. DOI: 10.1152/jn.1999.81.3.977. PMID: 10085326.
Article31. Wolfart J, Roeper J. 2002; Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J Neurosci. 22:3404–3413. Erratum in: J Neurosci. 2002;22:5250. DOI: 10.1523/JNEUROSCI.22-09-03404.2002. PMID: 11978817. PMCID: PMC6758365.
Article32. Wolfart J, Neuhoff H, Franz O, Roeper J. 2001; Differential expression of the small-conductance, calcium-activated potassium channel SK3 is critical for pacemaker control in dopaminergic midbrain neurons. J Neurosci. 21:3443–3456. DOI: 10.1523/JNEUROSCI.21-10-03443.2001. PMID: 11331374. PMCID: PMC6762487.
Article33. Deignan J, Luján R, Bond C, Riegel A, Watanabe M, Williams JT, Maylie J, Adelman JP. 2012; SK2 and SK3 expression differentially affect firing frequency and precision in dopamine neurons. Neuroscience. 217:67–76. DOI: 10.1016/j.neuroscience.2012.04.053. PMID: 22554781. PMCID: PMC3383402.
Article34. Kuznetsov AS, Kopell NJ, Wilson CJ. 2006; Transient high-frequency firing in a coupled-oscillator model of the mesencephalic dopaminergic neuron. J Neurophysiol. 95:932–947. DOI: 10.1152/jn.00691.2004. PMID: 16207783.
Article35. Chuhma N, Zhang H, Masson J, Zhuang X, Sulzer D, Hen R, Rayport S. 2004; Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. J Neurosci. 24:972–981. DOI: 10.1523/JNEUROSCI.4317-03.2004. PMID: 14749442. PMCID: PMC6729804.
Article36. Fiorillo CD, Williams JT. 1998; Glutamate mediates an inhibitory postsynaptic potential in dopamine neurons. Nature. 394:78–82. DOI: 10.1038/27919. PMID: 9665131.
Article37. Kim SH, Choi YM, Chung S, Uhm DY, Park MK. 2004; Two different Ca2+-dependent inhibitory mechanisms of spontaneous firing by glutamate in dopamine neurons. J Neurochem. 91:983–995. DOI: 10.1111/j.1471-4159.2004.02783.x. PMID: 15525352.
Article38. Morikawa H, Imani F, Khodakhah K, Williams JT. 2000; Inositol 1,4,5-triphosphate-evoked responses in midbrain dopamine neurons. J Neurosci. 20:RC103. DOI: 10.1523/JNEUROSCI.20-20-j0003.2000. PMID: 11027254. PMCID: PMC6772861.
Article39. Berridge MJ, Bootman MD, Roderick HL. 2003; Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 4:517–529. DOI: 10.1038/nrm1155. PMID: 12838335.
Article40. Papaioannou VE, Verkerk AO, Amin AS, de Bakker JM. 2013; Intracardiac origin of heart rate variability, pacemaker funny current and their possible association with critical illness. Curr Cardiol Rev. 9:82–96. DOI: 10.2174/1573403X11309010010.
Article41. Yaniv Y, Lyashkov AE, Sirenko S, Okamoto Y, Guiriba TR, Ziman BD, Morrell CH, Lakatta EG. 2014; Stochasticity intrinsic to coupled-clock mechanisms underlies beat-to-beat variability of spontaneous action potential firing in sinoatrial node pacemaker cells. J Mol Cell Cardiol. 77:1–10. DOI: 10.1016/j.yjmcc.2014.09.008. PMID: 25257916. PMCID: PMC4312254.
Article42. Anwar H, Hepburn I, Nedelescu H, Chen W, De Schutter E. 2013; Stochastic calcium mechanisms cause dendritic calcium spike variability. J Neurosci. 33:15848–15867. DOI: 10.1523/JNEUROSCI.1722-13.2013. PMID: 24089492. PMCID: PMC6618479.
Article43. Deister CA, Teagarden MA, Wilson CJ, Paladini CA. 2009; An intrinsic neuronal oscillator underlies dopaminergic neuron bursting. J Neurosci. 29:15888–15897. DOI: 10.1523/JNEUROSCI.4053-09.2009. PMID: 20016105. PMCID: PMC2824818.
Article44. Kuznetsova AY, Huertas MA, Kuznetsov AS, Paladini CA, Canavier CC. 2010; Regulation of firing frequency in a computational model of a midbrain dopaminergic neuron. J Comput Neurosci. 28:389–403. DOI: 10.1007/s10827-010-0222-y. PMID: 20217204. PMCID: PMC2929809.
Article45. Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. 2008; Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci. 11:178–186. DOI: 10.1038/nn2040. PMID: 18204443.
Article46. Debanne D, Campanac E, Bialowas A, Carlier E, Alcaraz G. 2011; Axon physiology. Physiol Rev. 91:555–602. DOI: 10.1152/physrev.00048.2009. PMID: 21527732.
Article47. Tucker KR, Huertas MA, Horn JP, Canavier CC, Levitan ES. 2012; Pacemaker rate and depolarization block in nigral dopamine neurons: a somatic sodium channel balancing act. J Neurosci. 32:14519–14531. DOI: 10.1523/JNEUROSCI.1251-12.2012. PMID: 23077037. PMCID: PMC3494994.
Article48. Rall W, Burke RE, Holmes WR, Jack JJ, Redman SJ, Segev I. 1992; Matching dendritic neuron models to experimental data. Physiol Rev. 72(4 Suppl):S159–S186. DOI: 10.1152/physrev.1992.72.suppl_4.S159. PMID: 1438585.
Article49. Migliore M, Shepherd GM. 2002; Emerging rules for the distributions of active dendritic conductances. Nat Rev Neurosci. 3:362–370. DOI: 10.1038/nrn810. PMID: 11988775.
Article50. Amini B, Clark JW Jr, Canavier CC. 1999; Calcium dynamics underlying pacemaker-like and burst firing oscillations in midbrain dopaminergic neurons: a computational study. J Neurophysiol. 82:2249–2261. DOI: 10.1152/jn.1999.82.5.2249. PMID: 10561403.
Article51. Katayama J, Akaike N, Nabekura J. 2003; Characterization of pre- and post-synaptic metabotropic glutamate receptor-mediated inhibitory responses in substantia nigra dopamine neurons. Neurosci Res. 45:101–115. DOI: 10.1016/S0168-0102(02)00202-X. PMID: 12507729.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regional difference in spontaneous firing inhibition by GABA(A) and GABA(B) receptors in nigral dopamine neurons
- In vitro generation of mature midbrain-type dopamine neurons by adjusting exogenous Nurr1 and Foxa2 expressions to their physiologic patterns
- Study on the Effect of Carbon Monoxide Intoxication to Serotonergic Neurons in Midbrain Raphe Nuclei of the Rat
- The Effect of Treatment with Tryptophan and/or Imipramine on the Serotonergic Immunoreactivity in Raphe Nucleus of Midbrain of the Rats
- Effects of 6-hydroxydopamine on the Adult Neurogenesis of Dopaminergic Neurons in the Mouse Midbrain