Korean J Physiol Pharmacol.
2024 Mar;28(2):121-127. 10.4196/kjpp.2024.28.2.121.
Development of a model to predict vancomycin serum concentration during continuous infusion of vancomycin in critically ill pediatric patients
- Affiliations
-
- 1Department of Pharmacy, Seoul National University Hospital, Seoul 03080, Korea
- 2Department of Pediatrics, Seoul National University Hospital and College of Medicine, Seoul 03080, Korea
- 3College of Pharmacy and Graduate School of Pharmaceutical Sciences, Ewha Womans University, Seoul 03760, Korea
- 4Innovative Medical Technology Research Institute, Seoul National University Hospital, Seoul 03080, Korea
- KMID: 2553524
- DOI: http://doi.org/10.4196/kjpp.2024.28.2.121
Abstract
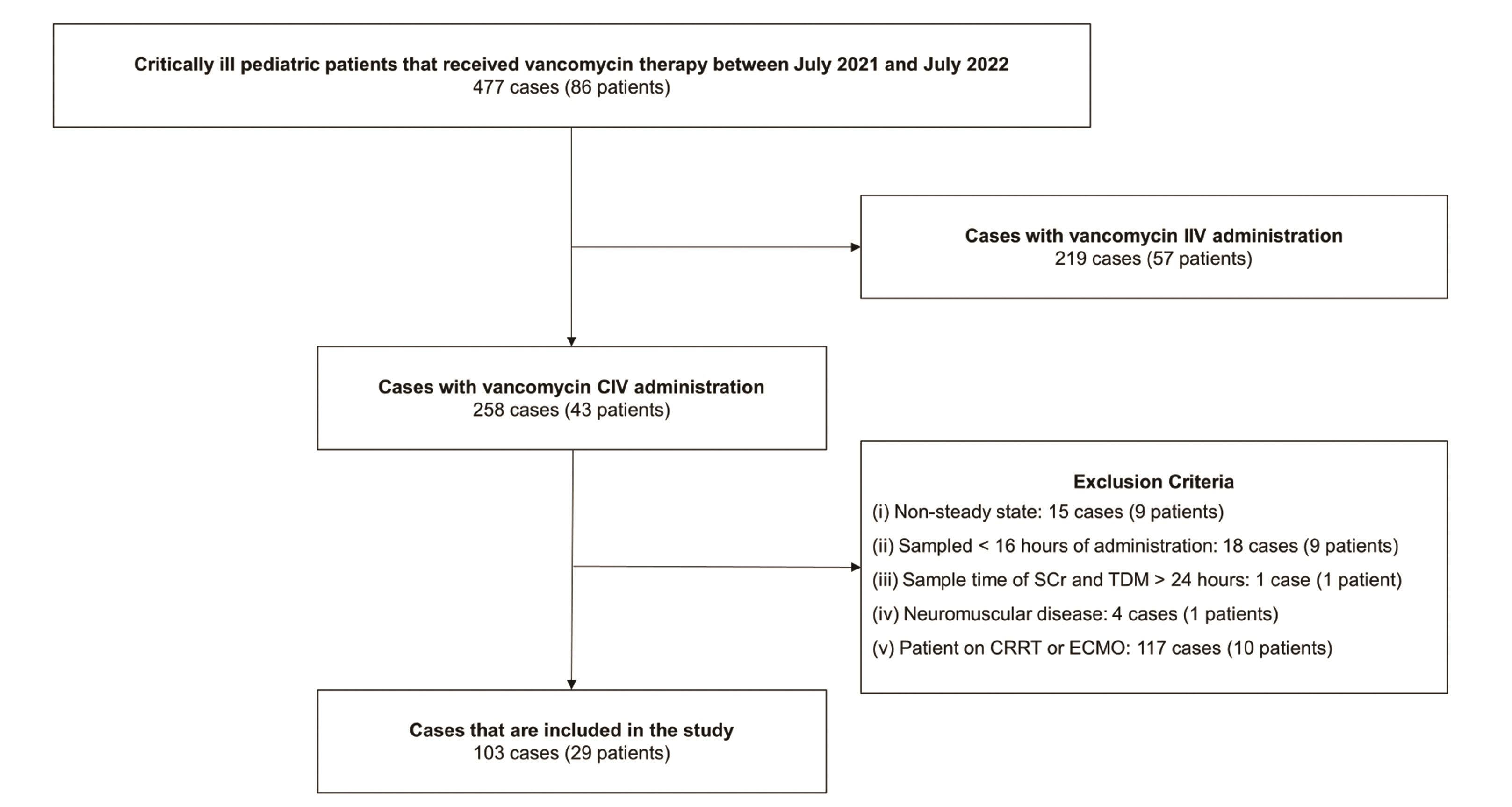
- Vancomycin is a frequently used antibiotic in intensive care units, and the patient’s renal clearance affects the pharmacokinetic characteristics of vancomycin. Several advantages have been reported for vancomycin continuous intravenous infusion, but studies on continuous dosing regimens based on patients’ renal clearance are insufficient. The aim of this study was to develop a vancomycin serum concentration prediction model by factoring in a patient’s renal clearance. Children admitted to our institution between July 1, 2021, and July 31, 2022 with records of continuous infusion of vancomycin were included in the study. Sex, age, height, weight, vancomycin dose by weight, interval from the start of vancomycin administration to the time of therapeutic drug monitoring sampling, and vancomycin serum concentrations were analyzed with the linear regression analysis of the mixed effect model. Univariable regression analysis was performed using the vancomycin serum concentration as a dependent variable. It showed that vancomycin dose (p < 0.001) and serum creatinine (p = 0.007) were factors that had the most impact on vancomycin serum concentration. Vancomycin serum concentration was affected by vancomycin dose (p < 0.001) and serum creatinine (p = 0.001) with statistical significance, and a multivariable regression model was obtained as follows: Vancomycin serum concentration (mg/l) = –1.296 + 0.281 × vancomycin dose (mg/kg) + 20.458 × serum creatinine (mg/dl) (adjusted coefficient of determination, R2 = 0.66). This prediction model is expected to contribute to establishing an optimal continuous infusion regimen for vancomycin.
Figure
Reference
-
1. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Talan DA, Chambers HF. J Rybak M. 2011; Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 52:285–292. DOI: 10.1093/cid/cir034. PMID: 21217178.
Article2. Wysocki M, Delatour F, Faurisson F, Rauss A, Pean Y, Misset B, Thomas F, Timsit JF, Similowski T, Mentec H, Mier L, Dreyfuss D. 2001; Continuous versus intermittent infusion of vancomycin in severe Staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother. 45:2460–2467. DOI: 10.1128/AAC.45.9.2460-2467.2001. PMID: 11502515. PMCID: PMC90678.
Article3. Hao JJ, Chen H, Zhou JX. 2016; Continuous versus intermittent infusion of vancomycin in adult patients: a systematic review and meta-analysis. Int J Antimicrob Agents. 47:28–35. DOI: 10.1016/j.ijantimicag.2015.10.019. PMID: 26655032.
Article4. Hong LT, Goolsby TA, Sherman DS, Mueller SW, Reynolds P, Cava L, Neumann R, Kiser TH. 2015; Continuous infusion vs intermittent vancomycin in neurosurgical intensive care unit patients. J Crit Care. 30:1153.e1–6. DOI: 10.1016/j.jcrc.2015.06.012. PMID: 26239323.
Article5. Roberts JA, Taccone FS, Udy AA, Vincent JL, Jacobs F, Lipman J. 2011; Vancomycin dosing in critically ill patients: robust methods for improved continuous-infusion regimens. Antimicrob Agents Chemother. 55:2704–2709. DOI: 10.1128/AAC.01708-10. PMID: 21402850. PMCID: PMC3101407.
Article6. Cristallini S, Hites M, Kabtouri H, Roberts JA, Beumier M, Cotton F, Lipman J, Jacobs F, Vincent JL, Creteur J, Taccone FS. 2016; New regimen for continuous infusion of vancomycin in critically ill patients. Antimicrob Agents Chemother. 60:4750–4756. DOI: 10.1128/AAC.00330-16. PMID: 27216073. PMCID: PMC4958221.
Article7. da Silva Alves GC, da Silva SD, Frade VP, Rodrigues D, Baldoni AO, de Castro WV, Sanches C. 2017; Determining the optimal vancomycin daily dose for pediatrics: a meta-analysis. Eur J Clin Pharmacol. 73:1341–1353. DOI: 10.1007/s00228-017-2306-3. PMID: 28776198.
Article8. Udy AA, Baptista JP, Lim NL, Joynt GM, Jarrett P, Wockner L, Boots RJ, Lipman J. 2014; Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations*. Crit Care Med. 42:520–527. DOI: 10.1097/CCM.0000000000000029. PMID: 24201175.9. Hurst AL, Baumgartner C, MacBrayne CE, Child J. 2019; Experience with continuous infusion vancomycin dosing in a large pediatric hospital. J Pediatric Infect Dis Soc. 8:174–179. DOI: 10.1093/jpids/piy032. PMID: 29718415.
Article10. McKamy S, Chen T, Lee M, Ambrose PJ. 2012; Evaluation of a pediatric continuous-infusion vancomycin therapy guideline. Am J Health Syst Pharm. 69:2066–2071. DOI: 10.2146/ajhp120072. PMID: 23172265.
Article11. Cies JJ, Moore WS 2nd, Conley SB, Muneeruddin S, Parker J, Shea P, Chopra A. 2016; Continuous infusion vancomycin through the addition of vancomycin to the continuous renal replacement therapy solution in the PICU: a case series. Pediatr Crit Care Med. 17:e138–e145. DOI: 10.1097/PCC.0000000000000656. PMID: 26890194.12. Genuini M, Oualha M, Bouazza N, Moulin F, Treluyer JM, Lesage F, Renolleau S, Benaboud S. 2018; Achievement of therapeutic vancomycin exposure with continuous infusion in critically ill children. Pediatr Crit Care Med. 19:e263–e269. DOI: 10.1097/PCC.0000000000001474. PMID: 29394210.
Article13. Guilhaumou R, Marsot A, Dupouey J, Galambrun C, Boulamery A, Coze C, Simon N, André N. 2016; Pediatric patients with solid or hematological tumor disease: vancomycin population pharmacokinetics and dosage optimization. Ther Drug Monit. 38:559–566. DOI: 10.1097/FTD.0000000000000318. PMID: 27631462.
Article14. Hoegy D, Goutelle S, Garnier N, Rénard C, Faure-Conter C, Bergeron C, Bertrand Y, Bleyzac N. 2018; Continuous intravenous vancomycin in children with normal renal function hospitalized in hematology-oncology: prospective validation of a dosing regimen optimizing steady-state concentration. Fundam Clin Pharmacol. 32:323–329. DOI: 10.1111/fcp.12344. PMID: 29315849.
Article15. Plan O, Cambonie G, Barbotte E, Meyer P, Devine C, Milesi C, Pidoux O, Badr M, Picaud JC. 2008; Continuous-infusion vancomycin therapy for preterm neonates with suspected or documented Gram-positive infections: a new dosage schedule. Arch Dis Child Fetal Neonatal Ed. 93:F418–F421. Erratum in: Arch Dis Child Fetal Neonatal Ed. 2009;94:F78. DOI: 10.1136/adc.2007.128280. PMID: 18450803.
Article16. Patel AD, Anand D, Lucas C, Thomson AH. 2013; Continuous infusion of vancomycin in neonates. Arch Dis Child. 98:478–479. DOI: 10.1136/archdischild-2012-303197. PMID: 23543265.
Article17. de Hoog M, Mouton JW, van den Anker JN. 2004; Vancomycin: pharmacokinetics and administration regimens in neonates. Clin Pharmacokinet. 43:417–440. DOI: 10.2165/00003088-200443070-00001. PMID: 15139793.18. Rainkie D, Ensom MH, Carr R. 2015; Pediatric assessment of vancomycin empiric dosing (PAVED): a retrospective review. Paediatr Drugs. 17:245–253. DOI: 10.1007/s40272-015-0122-8. PMID: 25813682.
Article19. Marsot A, Boulamery A, Bruguerolle B, Simon N. 2012; Vancomycin: a review of population pharmacokinetic analyses. Clin Pharmacokinet. 51:1–13. DOI: 10.2165/11596390-000000000-00000. PMID: 22149255.20. Stewart JJ, Jorgensen SC, Dresser L, Lau TT, Gin A, Thirion DJ, Nishi C, Dalton B. 2021; A Canadian perspective on the revised 2020 ASHP-IDSA-PIDS-SIDP guidelines for vancomycin AUC-based therapeutic drug monitoring for serious MRSA infections. J Assoc Med Microbiol Infect Dis Can. 6:3–9. DOI: 10.3138/jammi-2020-0028. PMID: 36340210. PMCID: PMC9612435.
Article21. Mould DR, Upton RN. 2012; Basic concepts in population modeling, simulation, and model-based drug development. CPT Pharmacometrics Syst Pharmacol. 1:e6. DOI: 10.1038/psp.2012.4. PMID: 23835886. PMCID: PMC3606044.
Article22. Spapen HD, Janssen van Doorn K, Diltoer M, Verbrugghe W, Jacobs R, Dobbeleir N, Honoré PM, Jorens PG. 2011; Retrospective evaluation of possible renal toxicity associated with continuous infusion of vancomycin in critically ill patients. Ann Intensive Care. 1:26. DOI: 10.1186/2110-5820-1-26. PMID: 21906376. PMCID: PMC3224465.
Article23. Saugel B, Gramm C, Wagner JY, Messer M, Lahmer T, Meidert AS, Schmid RM, Huber W. 2014; Evaluation of a dosing regimen for continuous vancomycin infusion in critically ill patients: an observational study in intensive care unit patients. J Crit Care. 29:351–355. DOI: 10.1016/j.jcrc.2013.12.007. PMID: 24456810.
Article24. Pea F, Furlanut M, Negri C, Pavan F, Crapis M, Cristini F, Viale P. 2009; Prospectively validated dosing nomograms for maximizing the pharmacodynamics of vancomycin administered by continuous infusion in critically ill patients. Antimicrob Agents Chemother. 53:1863–1867. DOI: 10.1128/AAC.01149-08. PMID: 19223642. PMCID: PMC2681515.
Article25. Buyle FM, Decruyenaere J, De Waele J, Tulkens PM, Van Audenrode T, Depuydt P, Claeys G, Robays H, Vogelaers D. 2013; A survey of beta-lactam antibiotics and vancomycin dosing strategies in intensive care units and general wards in Belgian hospitals. Eur J Clin Microbiol Infect Dis. 32:763–768. DOI: 10.1007/s10096-012-1803-7. PMID: 23271675.
Article26. Schlobohm CJ, Zhu E, Duby JJ. 2021; Continuous infusion versus intermittent infusion vancomycin in a burn center intensive care unit. Burns. 47:1495–1501. DOI: 10.1016/j.burns.2021.08.016. PMID: 34538672.
Article27. Rello J, Sole-Violan J, Sa-Borges M, Garnacho-Montero J, Muñoz E, Sirgo G, Olona M, Diaz E. 2005; Pneumonia caused by oxacillin-resistant Staphylococcus aureus treated with glycopeptides. Crit Care Med. 33:1983–1987. DOI: 10.1097/01.CCM.0000178180.61305.1D. PMID: 16148469.
Article28. Waineo MF, Kuhn TC, Brown DL. 2015; The pharmacokinetic/pharmacodynamic rationale for administering vancomycin via continuous infusion. J Clin Pharm Ther. 40:259–265. DOI: 10.1111/jcpt.12270. PMID: 25865426.
Article29. Ingram PR, Lye DC, Tambyah PA, Goh WP, Tam VH, Fisher DA. 2008; Risk factors for nephrotoxicity associated with continuous vancomycin infusion in outpatient parenteral antibiotic therapy. J Antimicrob Chemother. 62:168–171. DOI: 10.1093/jac/dkn080. PMID: 18334494.30. Cataldo MA, Tacconelli E, Grilli E, Pea F, Petrosillo N. 2012; Continuous versus intermittent infusion of vancomycin for the treatment of Gram-positive infections: systematic review and meta-analysis. J Antimicrob Chemother. 67:17–24. DOI: 10.1093/jac/dkr442. PMID: 22028203.
Article31. Akers KS, Cota JM, Chung KK, Renz EM, Mende K, Murray CK. 2012; Serum vancomycin levels resulting from continuous or intermittent infusion in critically ill burn patients with or without continuous renal replacement therapy. J Burn Care Res. 33:e254–e262. DOI: 10.1097/BCR.0b013e31825042fa. PMID: 22878490.
Article32. Covajes C, Scolletta S, Penaccini L, Ocampos-Martinez E, Abdelhadii A, Beumier M, Jacobs F, de Backer D, Vincent JL, Taccone FS. 2013; Continuous infusion of vancomycin in septic patients receiving continuous renal replacement therapy. Int J Antimicrob Agents. 41:261–266. DOI: 10.1016/j.ijantimicag.2012.10.018. PMID: 23312601.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vancomycin Dosing in Critically Ill Patients Receiving Continuous Renal Replacement Therapy
- Vancomycin-induced Hypersensitivity Reaction with Slow Infusion : A case report
- Vancomycin Pharmacokinetics in Oliguric Patients Undergoing Continuous Venovenous Hemodialysis and Continuous Venovenous Hemodiafiltration
- Evaluation of the Effect of Initial dose of Vancomycin using Serum Cystatin C as a Marker in Elderly Patients
- Differences in Vancomycin Clearance between Trauma and Medical Intensive Care Unit Patients