J Pathol Transl Med.
2024 Mar;58(2):49-58. 10.4132/jptm.2024.01.31.
Exploring histological predictive biomarkers for immune checkpoint inhibitor therapy response in non–small cell lung cancer
- Affiliations
-
- 1Department of Pathology, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- 2Division of Medical Oncology, Department of Internal Medicine, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- KMID: 2553472
- DOI: http://doi.org/10.4132/jptm.2024.01.31
Abstract
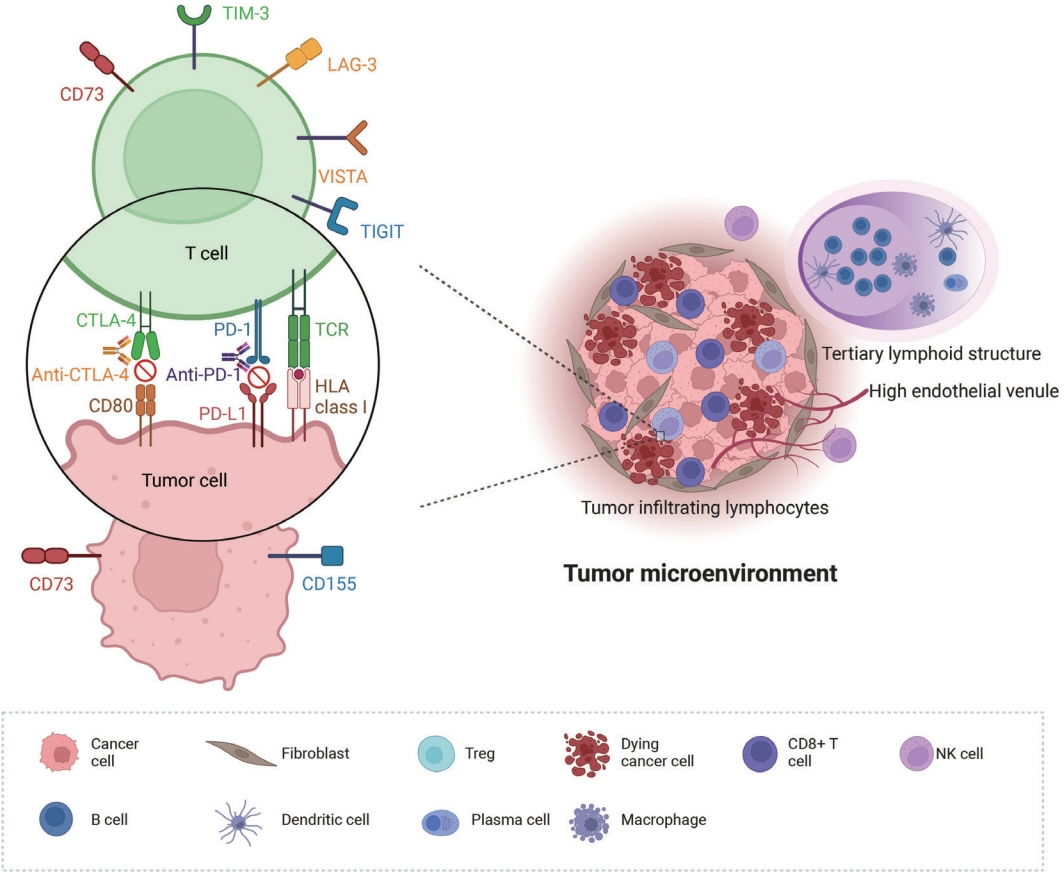
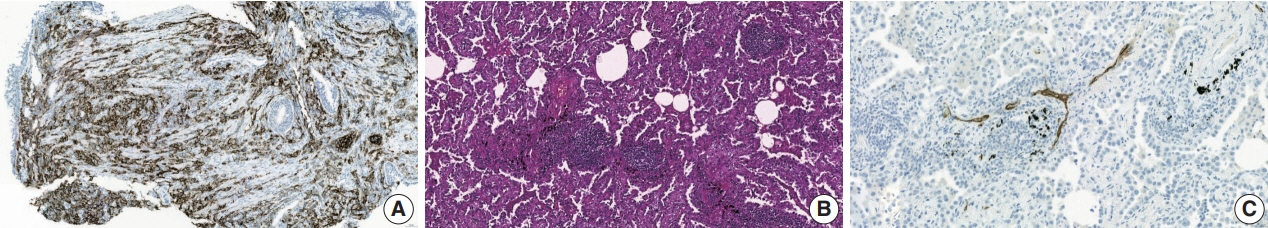
- Treatment challenges persist in advanced lung cancer despite the development of therapies beyond the traditional platinum-based chemotherapy. The early 2000s marked a shift to tyrosine kinase inhibitors targeting epidermal growth factor receptor, ushering in personalized genetic-based treatment. A further significant advance was the development of immune checkpoint inhibitors (ICIs), especially for non–small cell lung cancer. These target programmed death-ligand 1 (PD-L1) and cytotoxic T lymphocyte antigen 4, which enhanced the immune response against tumor cells. However, not all patients respond, and immune-related toxicities arise. This review emphasizes identifying biomarkers for ICI response prediction. While PD-L1 is a widely used, validated biomarker, its predictive accuracy is imperfect. Investigating tumor-infiltrating lymphocytes, tertiary lymphoid structure, and emerging biomarkers such as high endothelial venule, Human leukocyte antigen class I, T-cell immunoreceptors with Ig and ITIM domains, and lymphocyte activation gene-3 counts is promising. Understanding and exploring additional predictive biomarkers for ICI response are crucial for enhancing patient stratification and overall care in lung cancer treatment.
Figure
Reference
-
References
1. Kim HC, Jung CY, Cho DG, et al. Clinical characteristics and prognostic factors of lung cancer in Korea: a pilot study of data from the Korean Nationwide Lung Cancer Registry. Tuberc Respir Dis (Seoul). 2019; 82:118–25.2. Vital Statistics Division, Statistics Korea; Shin HY, Kim J, et al. Cause-of-death statistics in 2018 in the Republic of Korea. J Korean Med Assoc. 2020; 63:286–97.3. Alexander M, Kim SY, Cheng H. Update 2020: management of non-small cell lung cancer. Lung. 2020; 198:897–907.4. Lee GW. Current advances in the treatment of lung cancer with immune checkpoint inhibitors. J Korean Med Assoc. 2021; 64:333–41.5. Anagnostou VK, Brahmer JR. Cancer immunotherapy: a future paradigm shift in the treatment of non-small cell lung cancer. Clin Cancer Res. 2015; 21:976–84.6. Mamdani H, Matosevic S, Khalid AB, Durm G, Jalal SI. Immunotherapy in lung cancer: current landscape and future directions. Front Immunol. 2022; 13:823618.7. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12:252–64.8. Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022; 20:497–530.9. Camidge DR, Doebele RC, Kerr KM. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat Rev Clin Oncol. 2019; 16:341–55.10. Suresh K, Naidoo J, Lin CT, Danoff S. Immune checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest. 2018; 154:1416–23.11. Bai R, Lv Z, Xu D, Cui J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res. 2020; 8:34.12. Uruga H, Mino-Kenudson M. Predictive biomarkers for response to immune checkpoint inhibitors in lung cancer: PD-L1 and beyond. Virchows Arch. 2021; 478:31–44.13. Pabst L, Lopes S, Bertrand B, et al. Prognostic and predictive biomarkers in the era of immunotherapy for lung cancer. Int J Mol Sci. 2023; 24:7577.14. Kim H, Chung JH. PD-L1 testing in non-small cell lung cancer: past, present, and future. J Pathol Transl Med. 2019; 53:199–206.15. Ullah A, Pulliam S, Karki NR, et al. PD-L1 over-expression varies in different subtypes of lung cancer: will this affect future therapies? Clin Pract. 2022; 12:653–71.16. Domblides C, Leroy K, Monnet I, et al. Efficacy of immune checkpoint inhibitors in lung sarcomatoid carcinoma. J Thorac Oncol. 2020; 15:860–6.17. Sharma J, Borczuk A, Liu H, et al. P2.01-056 Distinct PD-L1 expression in different components of pulmonary sarcomatoid carcinoma and its association with MET mutation. Topic: immune mechanisms in thoracic cancer and targeted therapy. J Thoracic Oncol. 2017; 12(1 Suppl):S819–20.18. Brueckl WM, Ficker JH, Zeitler G. Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC). BMC Cancer. 2020; 20:1185.19. Li H, van der Merwe PA, Sivakumar S. Biomarkers of response to PD-1 pathway blockade. Br J Cancer. 2022; 126:1663–75.20. Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016; 34:2980–7.21. Yi M, Jiao D, Xu H, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018; 17:129.22. Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017; 12:208–22.23. Choi S, Cho SI, Ma M, et al. Artificial intelligence-powered programmed death ligand 1 analyser reduces interobserver variation in tumour proportion score for non-small cell lung cancer with better prediction of immunotherapy response. Eur J Cancer. 2022; 170:17–26.24. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014; 515:568–71.25. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019; 51:27–41.26. Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012; 12:298–306.27. Liu X, Hogg GD, DeNardo DG. Rethinking immune checkpoint blockade: ‘beyond the T cell’. J Immunother Cancer. 2021; 9:e001460.28. El Bairi K, Haynes HR, Blackley E, et al. The tale of TILs in breast cancer: a report from The International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer. 2021; 7:150.29. Aaltomaa S, Lipponen P, Eskelinen M, et al. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992; 28A:859–64.30. Luen SJ, Savas P, Fox SB, Salgado R, Loi S. Tumour-infiltrating lymphocytes and the emerging role of immunotherapy in breast cancer. Pathology. 2017; 49:141–55.31. Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010; 28:105–13.32. Salgado R, Denkert C, Demaria S, et al. The evaluation of tumorinfiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015; 26:259–71.33. Fuchs TL, Sioson L, Sheen A, et al. Assessment of tumor-infiltrating lymphocytes using International TILs Working Group (ITWG) system is a strong predictor of overall survival in colorectal carcinoma: a study of 1034 patients. Am J Surg Pathol. 2020; 44:536–44.34. Ahn SG, Jeong J, Hong S, Jung WH. Current issues and clinical evidence in tumor-infiltrating lymphocytes in breast cancer. J Pathol Transl Med. 2015; 49:355–63.35. Ali HR, Provenzano E, Dawson SJ, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014; 25:1536–43.36. Raskov H, Orhan A, Christensen JP, Gogenur I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br J Cancer. 2021; 124:359–67.37. Speiser DE, Chijioke O, Schaeuble K, Munz C. CD4(+) T cells in cancer. Nat Cancer. 2023; 4:317–29.38. Tay RE, Richardson EK, Toh HC. Revisiting the role of CD4(+) T cells in cancer immunotherapy: new insights into old paradigms. Cancer Gene Ther. 2021; 28:5–17.39. Gataa I, Mezquita L, Rossoni C, et al. Tumour-infiltrating lymphocyte density is associated with favourable outcome in patients with advanced non-small cell lung cancer treated with immunotherapy. Eur J Cancer. 2021; 145:221–9.40. Lopez de Rodas M, Nagineni V, Ravi A, et al. Role of tumor infiltrating lymphocytes and spatial immune heterogeneity in sensitivity to PD-1 axis blockers in non-small cell lung cancer. J Immunother Cancer. 2022; 10:e004440.41. Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015; 75:2139–45.42. Jiang Z, Zhou Y, Huang J. A combination of biomarkers predict response to immune checkpoint blockade therapy in non-small cell lung cancer. Front Immunol. 2021; 12:813331.43. Deutsch JS, Lipson EJ, Danilova L, et al. Combinatorial biomarker for predicting outcomes to anti-PD-1 therapy in patients with metastatic clear cell renal cell carcinoma. Cell Rep Med. 2023; 4:100947.44. Swisher SK, Wu Y, Castaneda CA, et al. Interobserver agreement between pathologists assessing tumor-infiltrating lymphocytes (TILs) in breast cancer using methodology proposed by the International TILs Working Group. Ann Surg Oncol. 2016; 23:2242–8.45. Chen DS, Mellman I. Elements of cancer immunity and the cancerimmune set point. Nature. 2017; 541:321–30.46. Park S, Ock CY, Kim H, et al. Artificial intelligence-powered spatial analysis of tumor-infiltrating lymphocytes as complementary biomarker for immune checkpoint inhibition in non-small-cell lung cancer. J Clin Oncol. 2022; 40:1916–28.47. Backman M, Strell C, Lindberg A, et al. Spatial immunophenotyping of the tumour microenvironment in non-small cell lung cancer. Eur J Cancer. 2023; 185:40–52.48. Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019; 19:307–25.49. Colbeck EJ, Ager A, Gallimore A, Jones GW. Tertiary lymphoid structures in cancer: drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease? Front Immunol. 2017; 8:1830.50. Trub M, Zippelius A. Tertiary lymphoid structures as a predictive biomarker of response to cancer immunotherapies. Front Immunol. 2021; 12:674565.51. Zhang Q, Wu S. Tertiary lymphoid structures are critical for cancer prognosis and therapeutic response. Front Immunol. 2022; 13:1063711.52. Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020; 577:549–55.53. Sautes-Fridman C, Verneau J, Sun CM, et al. Tertiary lymphoid structures and B cells: clinical impact and therapeutic modulation in cancer. Semin Immunol. 2020; 48:101406.54. Fridman WH, Petitprez F, Meylan M, et al. B cells and cancer: to B or not to B? J Exp Med. 2021; 218:e20200851.55. Silina K, Rulle U, Kalnina Z, Line A. Manipulation of tumour-infiltrating B cells and tertiary lymphoid structures: a novel anti-cancer treatment avenue? Cancer Immunol Immunother. 2014; 63:643–62.56. Rakaee M, Kilvaer TK, Jamaly S, et al. Tertiary lymphoid structure score: a promising approach to refine the TNM staging in resected non-small cell lung cancer. Br J Cancer. 2021; 124:1680–9.57. Blanchard L, Girard JP. High endothelial venules (HEVs) in immunity, inflammation and cancer. Angiogenesis. 2021; 24:719–53.58. Hong SA, Hwang HW, Kim MK, et al. High endothelial venule with concomitant high CD8+ tumor-infiltrating lymphocytes is associated with a favorable prognosis in resected gastric cancer. J Clin Med. 2020; 9:2628.59. Fang J, Lu Y, Zheng J, et al. Exploring the crosstalk between endothelial cells, immune cells, and immune checkpoints in the tumor microenvironment: new insights and therapeutic implications. Cell Death Dis. 2023; 14:586.60. Milutinovic S, Abe J, Godkin A, Stein JV, Gallimore A. The dual role of high endothelial venules in cancer progression versus immunity. Trends Cancer. 2021; 7:214–25.61. Allen E, Jabouille A, Rivera LB, et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 2017; 9:eaak9679.62. Ye D, Jin Y, Weng Y, et al. High endothelial venules predict response to PD-1 inhibitors combined with anti-angiogenesis therapy in NSCLC. Sci Rep. 2023; 13:16468.63. Hussain B, Kasinath V, Ashton-Rickardt GP, et al. High endothelial venules as potential gateways for therapeutics. Trends Immunol. 2022; 43:728–40.64. Hazini A, Fisher K, Seymour L. Deregulation of HLA-I in cancer and its central importance for immunotherapy. J Immunother Cancer. 2021; 9:e002899.65. Pagliuca S, Gurnari C, Rubio MT, Visconte V, Lenz TL. Individual HLA heterogeneity and its implications for cellular immune evasion in cancer and beyond. Front Immunol. 2022; 13:944872.66. Wang C, Xiong C, Hsu YC, Wang X, Chen L. Human leukocyte antigen (HLA) and cancer immunotherapy: HLA-dependent and -independent adoptive immunotherapies. Ann Blood. 2020; 5:14.67. Chowell D, Morris LG, Grigg CM, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2018; 359:582–7.68. Wang H, Liu B, Wei J. Beta2-microglobulin(B2M) in cancer immunotherapies: Biological function, resistance and remedy. Cancer Lett. 2021; 517:96–104.69. Gavrielatou N, Vathiotis I, Aung TN, et al. Digital spatial profiling links beta-2-microglobulin expression with immune checkpoint blockade outcomes in head and neck squamous cell carcinoma. Cancer Res Commun. 2023; 3:558–63.70. Sade-Feldman M, Jiao YJ, Chen JH, et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun. 2017; 8:1136.71. Lechner MG, Karimi SS, Barry-Holson K, et al. Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J Immunother. 2013; 36:477–89.72. Sabbatino F, Liguori L, Polcaro G, et al. Role of human leukocyte antigen system as a predictive biomarker for checkpoint-based immunotherapy in cancer patients. Int J Mol Sci. 2020; 21:7295.73. Ashizawa T, Iizuka A, Nonomura C, et al. Antitumor effect of programmed death-1 (PD-1) blockade in humanized the NOG-MHC double knockout mouse. Clin Cancer Res. 2017; 23:149–58.74. Ladanyi A, Hegyi B, Balatoni T, et al. HLA class I downregulation in progressing metastases of melanoma patients treated with ipilimumab. Pathol Oncol Res. 2022; 28:1610297.75. Pescia C, Pini G, Olmeda E, Ferrero S, Lopez G. TIGIT in lung cancer: potential theranostic implications. Life (Basel). 2023; 13:1050.76. Yang Z, Peng Y, Xu J, et al. PVR/TIGIT and PD-L1/PD-1 expression predicts survival and enlightens combined immunotherapy in lung squamous cell carcinoma. Transl Oncol. 2022; 24:101501.77. Kim SW, Kim YI, Mustafa B, et al. Distribution pattern of tumor infiltrating lymphocytes and tumor microenvironment composition as prognostic indicators in anorectal malignant melanoma. Mod Pathol. 2021; 34:141–60.78. Aggarwal V, Workman CJ, Vignali DA. LAG-3 as the third checkpoint inhibitor. Nat Immunol. 2023; 24:1415–22.79. Sauer N, Szlasa W, Jonderko L, et al. LAG-3 as a potent target for novel anticancer therapies of a wide range of tumors. Int J Mol Sci. 2022; 23:9958.80. He Y, Yu H, Rozeboom L, et al. LAG-3 protein expression in nonsmall cell lung cancer and its relationship with PD-1/PD-L1 and tumor-infiltrating lymphocytes. J Thorac Oncol. 2017; 12:814–23.81. Borgeaud M, Sandoval J, Obeid M, et al. Novel targets for immunecheckpoint inhibition in cancer. Cancer Treat Rev. 2023; 120:102614.82. Inoue Y, Yoshimura K, Kurabe N, et al. Prognostic impact of CD73 and A2A adenosine receptor expression in non-small-cell lung cancer. Oncotarget. 2017; 8:8738–51.83. Loi S, Pommey S, Haibe-Kains B, et al. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A. 2013; 110:11091–6.84. Wu XR, He XS, Chen YF, et al. High expression of CD73 as a poor prognostic biomarker in human colorectal cancer. J Surg Oncol. 2012; 106:130–7.85. Rocha P, Salazar R, Zhang J, et al. CD73 expression defines immune, molecular, and clinicopathological subgroups of lung adenocarcinoma. Cancer Immunol Immunother. 2021; 70:1965–76.86. Bendell JC, LoRusso P, Overman MJ, et al. Safety and efficacy of the anti-CD73 monoclonal antibody (mAb) oleclumab ± durvalumab in patients (pts) with advanced colorectal cancer (CRC), pancreatic ductal adenocarcinoma (PDAC), or EGFR-mutant non-small cell lung cancer (EGFRm NSCLC). J Clin Oncol. 2021; 39:9047.87. Jin D, Fan J, Wang L, et al. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010; 70:2245–55.88. Ohta A, Gorelik E, Prasad SJ, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006; 103:13132–7.89. Ishii H, Azuma K, Kawahara A, et al. Predictive value of CD73 expression for the efficacy of immune checkpoint inhibitors in NSCLC. Thorac Cancer. 2020; 11:950–5.90. Lee JB, Ha SJ, Kim HR. Clinical insights into novel immune checkpoint inhibitors. Front Pharmacol. 2021; 12:681320.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunotherapy for Non-Small Cell Lung Cancer
- Treatment of advanced urogenital cancers with immune checkpoint inhibitors
- Current advances in the treatment of lung cancer with immune checkpoint inhibitors
- Immunotherapy for Non-small-cell Lung Cancer: Current Status and Future Obstacles
- Gut microbiome on immune checkpoint inhibitor therapy and consequent immune-related colitis: a review