Clin Exp Otorhinolaryngol.
2024 Feb;17(1):1-14. 10.21053/ceo.2023.01382.
Hair Cell Regeneration: From Animals to Humans
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Stanford University School of Medicine, Stanford, CA, USA
- 2Department of Otorhinolaryngology-Head and Neck Surgery and Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Pusan National University School of Medicine, Busan, Korea
- KMID: 2553051
- DOI: http://doi.org/10.21053/ceo.2023.01382
Abstract
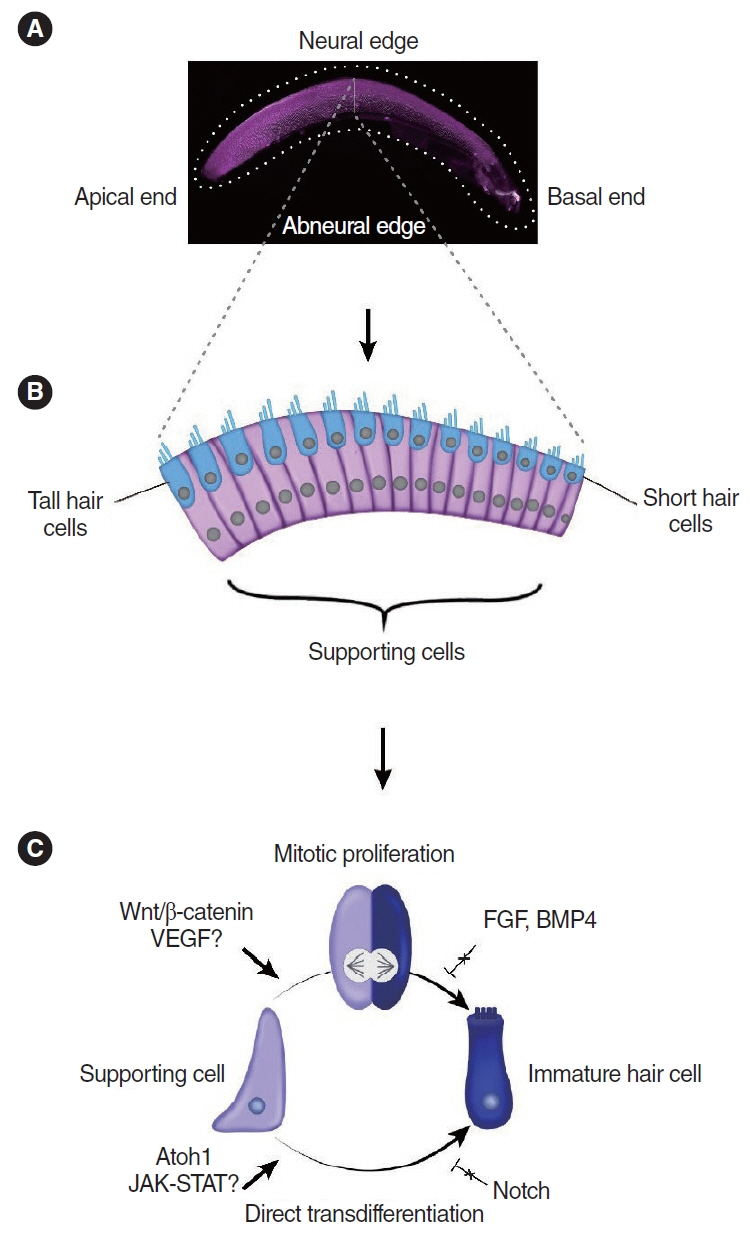
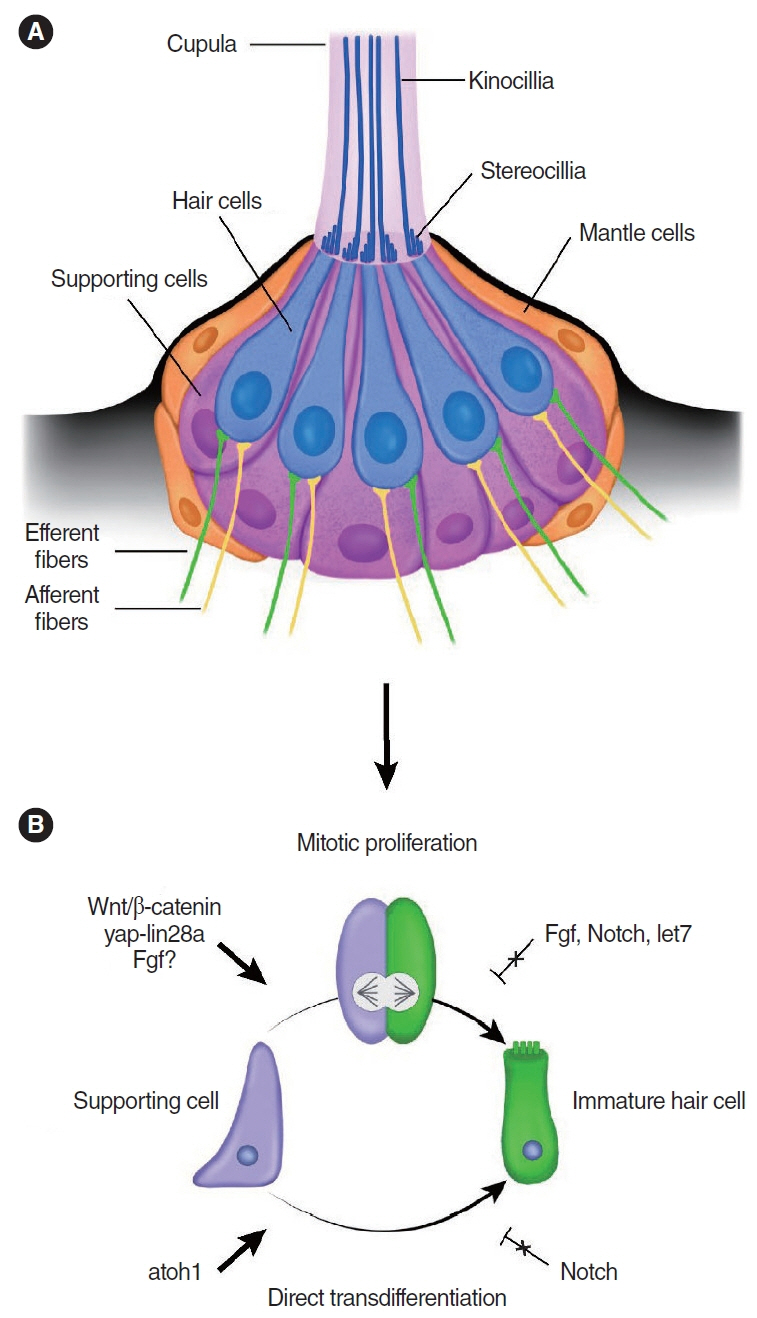
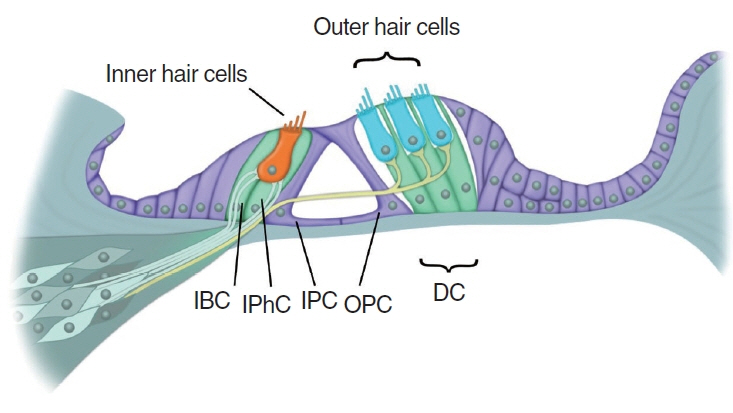
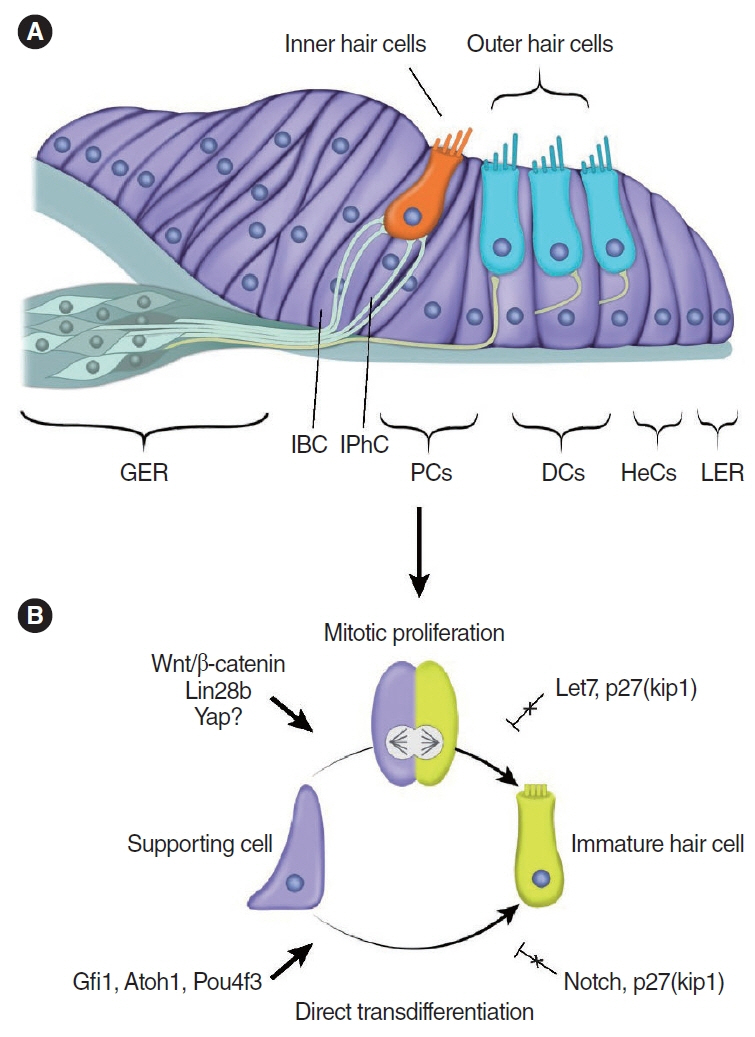
- Cochlear hair cells convert sound into electrical signals that are relayed via the spiral ganglion neurons to the central auditory pathway. Hair cells are vulnerable to damage caused by excessive noise, aging, and ototoxic agents. Non-mammals can regenerate lost hair cells by mitotic regeneration and direct transdifferentiation of surrounding supporting cells. However, in mature mammals, damaged hair cells are not replaced, resulting in permanent hearing loss. Recent studies have uncovered mechanisms by which sensory organs in non-mammals and the neonatal mammalian cochlea regenerate hair cells, and outlined possible mechanisms why this ability declines rapidly with age in mammals. Here, we review similarities and differences between avian, zebrafish, and mammalian hair cell regeneration. Moreover, we discuss advances and limitations of hair cell regeneration in the mature cochlea and their potential applications to human hearing loss.
Keyword
Figure
Reference
-
1. Chadha S, Kamenov K, Cieza A. The world report on hearing, 2021. Bull World Health Organ. 2021; Apr. 99(4):242–A.2. Wu PZ, O’Malley JT, de Gruttola V, Liberman MC. Age-related hearing loss is dominated by damage to inner ear sensory cells, not the cellular battery that powers them. J Neurosci. 2020; Aug. 40(33):6357–66.3. Atkinson PJ, Huarcaya Najarro E, Sayyid ZN, Cheng AG. Sensory hair cell development and regeneration: similarities and differences. Development. 2015; May. 142(9):1561–71.4. Hudspeth AJ, Choe Y, Mehta AD, Martin P. Putting ion channels to work: mechanoelectrical transduction, adaptation, and amplification by hair cells. Proc Natl Acad Sci U S A. 2000; Oct. 97(22):11765–72.5. Denans N, Baek S, Piotrowski T. Comparing sensory organs to define the path for hair cell regeneration. Annu Rev Cell Dev Biol. 2019; Oct. 35:567–89.6. Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006; Nov. 7(11):837–49.7. Bohne BA, Ward PH, Fernandez C. Irreversible inner ear damage from rock music. Trans Sect Otolaryngol Am Acad Ophthalmol Otolaryngol. 1976; 82(1):ORL50–9.8. Oesterle EC, Bhave SA, Coltrera MD. Basic fibroblast growth factor inhibits cell proliferation in cultured avian inner ear sensory epithelia. J Comp Neurol. 2000; Aug. 424(2):307–26.9. Hawkins JE. Microcirculation in the labyrinth. Arch Otorhinolaryngol. 1976; Sep. 212(4):241–51.10. Brigande JV, Heller S. Quo vadis, hair cell regeneration. Nat Neurosci. 2009; Jun. 12(6):679–85.11. Malgrange B, Belachew S, Thiry M, Nguyen L, Rogister B, Alvarez ML, et al. Proliferative generation of mammalian auditory hair cells in culture. Mech Dev. 2002; Mar. 112(1-2):79–88.12. Sinkkonen ST, Chai R, Jan TA, Hartman BH, Laske RD, Gahlen F, et al. Intrinsic regenerative potential of murine cochlear supporting cells. Sci Rep. 2011; 1:26.13. Cox BC, Chai R, Lenoir A, Liu Z, Zhang L, Nguyen DH, et al. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development. 2014; Feb. 141(4):816–29.14. White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006; Jun. 441(7096):984–7.15. Corwin JT, Oberholtzer JC. Fish n’ chicks: model recipes for hair-cell regeneration. Neuron. 1997; Nov. 19(5):951–4.16. Brignull HR, Raible DW, Stone JS. Feathers and fins: non-mammalian models for hair cell regeneration. Brain Res. 2009; Jun. 1277:12–23.17. Rubel EW, Furrer SA, Stone JS. A brief history of hair cell regeneration research and speculations on the future. Hear Res. 2013; Mar. 297:42–51.18. Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988; Jun. 240(4860):1772–4.19. Stone JS, Rubel EW. Delta1 expression during avian hair cell regeneration. Development. 1999; Feb. 126(5):961–73.20. Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988; Jun. 240(4860):1774–6.21. Cruz RM, Lambert PR, Rubel EW. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch Otolaryngol Head Neck Surg. 1987; Oct. 113(10):1058–62.22. Froymovich O, Rebala V, Salvi RJ, Rassael H. Long-term effect of acoustic trauma on distortion product otoacoustic emissions in chickens. J Acoust Soc Am. 1995; May. 97(5 Pt 1):3021–9.23. Poje CP, Sewell DA, Saunders JC. The effects of exposure to intense sound on the DC endocochlear potential in the chick. Hear Res. 1995; Feb. 82(2):197–204.24. Cruz IA, Kappedal R, Mackenzie SM, Hailey DW, Hoffman TL, Schilling TF, et al. Robust regeneration of adult zebrafish lateral line hair cells reflects continued precursor pool maintenance. Dev Biol. 2015; Jun. 402(2):229–38.25. Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). J Assoc Res Otolaryngol. 2003; Jun. 4(2):219–34.26. Williams JA, Holder N. Cell turnover in neuromasts of zebrafish larvae. Hear Res. 2000; May. 143(1-2):171–81.27. Hernandez PP, Moreno V, Olivari FA, Allende ML. Sub-lethal concentrations of waterborne copper are toxic to lateral line neuromasts in zebrafish (Danio rerio). Hear Res. 2006; Mar. 213(1-2):1–10.28. Stone JS, Cotanche DA. Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol. 2007; 51(6-7):633–47.29. Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008; Feb. 28(9):2261–73.30. Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci Lett. 1996; Feb. 205(1):17–20.31. Roberson DW, Kreig C, Rubel EW. Light microscopic evidence that direct transdifferentiation gives rise to new hair cells in regenerating avian auditory epithelium. Audit Neurosci. 1996; 2:195–205.32. Hernandez PP, Olivari FA, Sarrazin AF, Sandoval PC, Allende ML. Regeneration in zebrafish lateral line neuromasts: expression of the neural progenitor cell marker sox2 and proliferation-dependent and-independent mechanisms of hair cell renewal. Dev Neurobiol. 2007; Apr. 67(5):637–54.33. Lopez-Schier H, Hudspeth AJ. A two-step mechanism underlies the planar polarization of regenerating sensory hair cells. Proc Natl Acad Sci U S A. 2006; Dec. 103(49):18615–20.34. Cafaro J, Lee GS, Stone JS. Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev Dyn. 2007; Jan. 236(1):156–70.35. Roberson DW, Alosi JA, Cotanche DA. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J Neurosci Res. 2004; Nov. 78(4):461–71.36. Warchol ME, Corwin JT. Regenerative proliferation in organ cultures of the avian cochlea: identification of the initial progenitors and determination of the latency of the proliferative response. J Neurosci. 1996; Sep. 16(17):5466–77.37. Gomez-Dorado M, Daudet N, Gale JE, Dawson SJ. Differential regulation of mammalian and avian ATOH1 by E2F1 and its implication for hair cell regeneration in the inner ear. Sci Rep. 2021; Sep. 11(1):19368.38. Baek S, Tran NT, Diaz DC, Tsai YY, Acedo JN, Lush ME, et al. Single-cell transcriptome analysis reveals three sequential phases of gene expression during zebrafish sensory hair cell regeneration. Dev Cell. 2022; Mar. 57(6):799–819.39. Janesick AS, Scheibinger M, Benkafadar N, Kirti S, Heller S. Avian auditory hair cell regeneration is accompanied by JAK/STAT-dependent expression of immune-related genes in supporting cells. Development. 2022; Apr. 149(8):dev200113.40. Matsunaga M, Kita T, Yamamoto R, Yamamoto N, Okano T, Omori K, et al. Initiation of supporting cell activation for hair cell regeneration in the avian auditory epithelium: an explant culture model. Front Cell Neurosci. 2020; 14:583994.41. Janesick A, Scheibinger M, Benkafadar N, Kirti S, Ellwanger DC, Heller S. Cell-type identity of the avian cochlea. Cell Rep. 2021; Mar. 34(12):108900.42. Benkafadar N, Janesick A, Scheibinger M, Ling AH, Jan TA, Heller S. Transcriptomic characterization of dying hair cells in the avian cochlea. Cell Rep. 2021; Mar. 34(12):108902.43. Ellwanger DC, Scheibinger M, Dumont RA, Barr-Gillespie PG, Heller S. Transcriptional dynamics of hair-bundle morphogenesis revealed with CellTrails. Cell Rep. 2018; Jun. 23(10):2901–14.44. Hawkins RD, Bashiardes S, Powder KE, Sajan SA, Bhonagiri V, Alvarado DM, et al. Large scale gene expression profiles of regenerating inner ear sensory epithelia. PLoS One. 2007; Jun. 2(6):e525.45. Alvarado DM, Hawkins RD, Bashiardes S, Veile RA, Ku YC, Powder KE, et al. An RNA interference-based screen of transcription factor genes identifies pathways necessary for sensory regeneration in the avian inner ear. J Neurosci. 2011; Mar. 31(12):4535–43.46. Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017; Apr. 77(5):521–46.47. Matsunaga M, Yamamoto R, Kita T, Ohnishi H, Yamamoto N, Okano T, et al. Stepwise fate conversion of supporting cells to sensory hair cells in the chick auditory epithelium. iScience. 2023; Feb. 26(2):106046.48. Lewis RM, Hume CR, Stone JS. Atoh1 expression and function during auditory hair cell regeneration in post-hatch chickens. Hear Res. 2012; Jul. 289(1-2):74–85.49. Bermingham-McDonogh O, Stone JS, Reh TA, Rubel EW. FGFR3 expression during development and regeneration of the chick inner ear sensory epithelia. Dev Biol. 2001; Oct. 238(2):247–59.50. Lewis RM, Keller JJ, Wan L, Stone JS. Bone morphogenetic protein 4 antagonizes hair cell regeneration in the avian auditory epithelium. Hear Res. 2018; Jul. 364:1–11.51. Daudet N, Gibson R, Shang J, Bernard A, Lewis J, Stone J. Notch regulation of progenitor cell behavior in quiescent and regenerating auditory epithelium of mature birds. Dev Biol. 2009; Feb. 326(1):86–100.52. Jacques BE, Montgomery WH, Uribe PM, Yatteau A, Asuncion JD, Resendiz G, et al. The role of Wnt/β-catenin signaling in proliferation and regeneration of the developing basilar papilla and lateral line. Dev Neurobiol. 2014; Apr. 74(4):438–56.53. Luo Z, Du Y, Li S, Zhang H, Shu M, Zhang D, et al. Three distinct Atoh1 enhancers cooperate for sound receptor hair cell development. Proc Natl Acad Sci U S A. 2022; Aug. 119(32):e2119850119.54. Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005; Feb. 132(3):541–51.55. Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, et al. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998; Dec. 125(23):4645–54.56. Mustonen T, Alitalo K. Endothelial receptor tyrosine kinases involved in angiogenesis. J Cell Biol. 1995; May. 129(4):895–8.57. Wan L, Lovett M, Warchol ME, Stone JS. Vascular endothelial growth factor is required for regeneration of auditory hair cells in the avian inner ear. Hear Res. 2020; Jan. 385:107839.58. Baxendale S, Whitfield TT. Methods to study the development, anatomy, and function of the zebrafish inner ear across the life course. Methods Cell Biol. 2016; 134:165–209.59. Pickett SB, Raible DW. Water waves to sound waves: using zebrafish to explore hair cell biology. J Assoc Res Otolaryngol. 2019; Feb. 20(1):1–19.60. Matsui JI, Ryals BM. Hair cell regeneration: an exciting phenomenon... but will restoring hearing and balance be possible. J Rehabil Res Dev. 2005; 42(4 Suppl 2):187–98.61. Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Malicki J, Stemple DL, et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development. 1996; Dec. 123:37–46.62. Teitz T, Fang J, Goktug AN, Bonga JD, Diao S, Hazlitt RA, et al. CDK2 inhibitors as candidate therapeutics for cisplatin- and noise-induced hearing loss. J Exp Med. 2018; Apr. 215(4):1187–203.63. Kimmel CB. Genetics and early development of zebrafish. Trends Genet. 1989; Aug. 5(8):283–8.64. Villegas R, Martin SM, O’Donnell KC, Carrillo SA, Sagasti A, Allende ML. Dynamics of degeneration and regeneration in developing zebrafish peripheral axons reveals a requirement for extrinsic cell types. Neural Dev. 2012; Jun. 7:19.65. Haehnel M, Taguchi M, Liao JC. Heterogeneity and dynamics of lateral line afferent innervation during development in zebrafish (Danio rerio). J Comp Neurol. 2012; May. 520(7):1376–86.66. Bever MM, Fekete DM. Atlas of the developing inner ear in zebrafish. Dev Dyn. 2002; Apr. 223(4):536–43.67. Venuto A, Erickson T. Evaluating the death and recovery of lateral line hair cells following repeated neomycin treatments. Life (Basel). 2021; Nov. 11(11):1180.68. Breitzler L, Lau IH, Fonseca PJ, Vasconcelos RO. Noise-induced hearing loss in zebrafish: investigating structural and functional inner ear damage and recovery. Hear Res. 2020; Jun. 391:107952.69. Millimaki BB, Sweet EM, Riley BB. Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Dev Biol. 2010; Feb. 338(2):262–9.70. Wibowo I, Pinto-Teixeira F, Satou C, Higashijima S, Lopez-Schier H. Compartmentalized Notch signaling sustains epithelial mirror symmetry. Development. 2011; Mar. 138(6):1143–52.71. Mackenzie SM, Raible DW. Proliferative regeneration of zebrafish lateral line hair cells after different ototoxic insults. PLoS One. 2012; 7(10):e47257.72. Pinto-Teixeira F, Viader-Llargues O, Torres-Mejia E, Turan M, Gonzalez-Gualda E, Pola-Morell L, et al. Inexhaustible hair-cell regeneration in young and aged zebrafish. Biol Open. 2015; May. 4(7):903–9.73. Thomas ED, Raible DW. Distinct progenitor populations mediate regeneration in the zebrafish lateral line. Elife. 2019; Mar. 8:e43736.74. Romero-Carvajal A, Navajas Acedo J, Jiang L, Kozlovskaja-Gumbriene A, Alexander R, Li H, et al. Regeneration of sensory hair cells requires localized interactions between the Notch and Wnt pathways. Dev Cell. 2015; Aug. 34(3):267–82.75. Mi XX, Yan J, Li Y, Shi JP. Wnt/β-catenin signaling was activated in supporting cells during exposure of the zebrafish lateral line to cisplatin. Ann Anat. 2019; Nov. 226:48–56.76. Jiang L, Romero-Carvajal A, Haug JS, Seidel CW, Piotrowski T. Gene-expression analysis of hair cell regeneration in the zebrafish lateral line. Proc Natl Acad Sci U S A. 2014; Apr. 111(14):E1383–92.77. Tang D, He Y, Li W, Li H. Wnt/β-catenin interacts with the FGF pathway to promote proliferation and regenerative cell proliferation in the zebrafish lateral line neuromast. Exp Mol Med. 2019; May. 51(5):1–16.78. Lush ME, Diaz DC, Koenecke N, Baek S, Boldt H, St Peter MK, et al. scRNA-Seq reveals distinct stem cell populations that drive hair cell regeneration after loss of Fgf and Notch signaling. Elife. 2019; Jan. 8:e44431.79. Ye Z, Su Z, Xie S, Liu Y, Wang Y, Xu X, et al. Yap-lin28a axis targets let7-Wnt pathway to restore progenitors for initiating regeneration. Elife. 2020; Apr. 9:e55771.80. Rybak A, Fuchs H, Smirnova L, Brandt C, Pohl EE, Nitsch R, et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol. 2008; Aug. 10(8):987–93.81. Golden EJ, Benito-Gonzalez A, Doetzlhofer A. The RNA-binding protein LIN28B regulates developmental timing in the mammalian cochlea. Proc Natl Acad Sci U S A. 2015; Jul. 112(29):E3864–73.82. Zhang R, Liu X, Li Y, Wang M, Chen L, Hu B. Suppression of inflammation delays hair cell regeneration and functional recovery following lateral line damage in zebrafish larvae. Biomolecules. 2020; Oct. 10(10):1451.83. Warchol ME, Schrader A, Sheets L. Macrophages respond rapidly to ototoxic injury of lateral line hair cells but are not required for hair cell regeneration. Front Cell Neurosci. 2020; Jan. 14:613246.84. Denans N, Tran NT, Swall ME, Diaz DC, Blanck J, Piotrowski T. An anti-inflammatory activation sequence governs macrophage transcriptional dynamics during tissue injury in zebrafish. Nat Commun. 2022; Sep. 13(1):5356.85. Kaur T, Zamani D, Tong L, Rubel EW, Ohlemiller KK, Hirose K, et al. Fractalkine signaling regulates macrophage recruitment into the cochlea and promotes the survival of spiral ganglion neurons after selective hair cell lesion. J Neurosci. 2015; Nov. 35(45):15050–61.86. Manickam V, Gawande DY, Stothert AR, Clayman AC, Batalkina L, Warchol ME, et al. Macrophages promote repair of inner hair cell ribbon synapses following noise-induced cochlear synaptopathy. J Neurosci. 2023; Mar. 43(12):2075–89.87. Fredelius L, Rask-Andersen H. The role of macrophages in the disposal of degeneration products within the organ of corti after acoustic overstimulation. Acta Otolaryngol. 1990; 109(1-2):76–82.88. Hirose K, Discolo CM, Keasler JR, Ransohoff R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J Comp Neurol. 2005; Aug. 489(2):180–94.89. Sato E, Shick HE, Ransohoff RM, Hirose K. Expression of fractalkine receptor CX3CR1 on cochlear macrophages influences survival of hair cells following ototoxic injury. J Assoc Res Otolaryngol. 2010; Jun. 11(2):223–34.90. Kolla L, Kelly MC, Mann ZF, Anaya-Rocha A, Ellis K, Lemons A, et al. Characterization of the development of the mouse cochlear epithelium at the single cell level. Nat Commun. 2020; May. 11(1):2389.91. Bramhall NF, Shi F, Arnold K, Hochedlinger K, Edge AS. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Reports. 2014; Mar. 2(3):311–22.92. Heuermann ML, Matos S, Hamilton D, Cox BC. Regenerated hair cells in the neonatal cochlea are innervated and the majority coexpress markers of both inner and outer hair cells. Front Cell Neurosci. 2022; Sep. 16:841864.93. Baker NE, Yu S, Han D. Evolution of proneural atonal expression during distinct regulatory phases in the developing Drosophila eye. Curr Biol. 1996; Oct. 6(10):1290–301.94. Chai R, Xia A, Wang T, Jan TA, Hayashi T, Bermingham-McDonogh O, et al. Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea. J Assoc Res Otolaryngol. 2011; Aug. 12(4):455–69.95. Shi F, Kempfle JS, Edge AS. Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J Neurosci. 2012; Jul. 32(28):9639–48.96. McGovern MM, Randle MR, Cuppini CL, Graves KA, Cox BC. Multiple supporting cell subtypes are capable of spontaneous hair cell regeneration in the neonatal mouse cochlea. Development. 2019; Development. 146(4):–dev171009.97. Zhang S, Liu D, Dong Y, Zhang Z, Zhang Y, Zhou H, et al. Frizzled-9+ supporting cells are progenitors for the generation of hair cells in the postnatal mouse cochlea. Front Mol Neurosci. 2019; 12:184.98. Lee S, Song JJ, Beyer LA, Swiderski DL, Prieskorn DM, Acar M, et al. Combinatorial Atoh1 and Gfi1 induction enhances hair cell regeneration in the adult cochlea. Sci Rep. 2020; Dec. 10(1):21397.99. Shu Y, Li W, Huang M, Quan YZ, Scheffer D, Tian C, et al. Renewed proliferation in adult mouse cochlea and regeneration of hair cells. Nat Commun. 2019; Dec. 10(1):5530.100. Walters BJ, Coak E, Dearman J, Bailey G, Yamashita T, Kuo B, et al. in vivo interplay between p27Kip1, GATA3, ATOH1, and POU4F3 converts non-sensory cells to hair cells in adult mice. Cell Rep. 2017; Apr. 19(2):307–20.101. Yamashita T, Zheng F, Finkelstein D, Kellard Z, Carter R, Rosencrance CD, et al. High-resolution transcriptional dissection of in vivo Atoh1-mediated hair cell conversion in mature cochleae identifies Isl1 as a co-reprogramming factor. PLoS Genet. 2018; Jul. 14(7):e1007552.102. Sun S, Li S, Luo Z, Ren M, He S, Wang G, et al. Dual expression of Atoh1 and Ikzf2 promotes transformation of adult cochlear supporting cells into outer hair cells. Elife. 2021; Sep. 10:e66547.103. Li XJ, Doetzlhofer A. LIN28B/let-7 control the ability of neonatal murine auditory supporting cells to generate hair cells through mTOR signaling. Proc Natl Acad Sci U S A. 2020; Sep. 117(36):22225–36.104. McLean WJ, Yin X, Lu L, Lenz DR, McLean D, Langer R, et al. Clonal expansion of Lgr5-positive cells from mammalian cochlea and high-purity generation of sensory hair cells. Cell Rep. 2017; Feb. 18(8):1917–29.105. Li XJ, Morgan C, Goff LA, Doetzlhofer A. Follistatin promotes LIN28B-mediated supporting cell reprogramming and hair cell regeneration in the murine cochlea. Sci Adv. 2022; Feb. 8(6):eabj7651.106. Pauklin S, Vallier L. Activin/Nodal signalling in stem cells. Development. 2015; Feb. 142(4):607–19.107. Tao L, Yu HV, Llamas J, Trecek T, Wang X, Stojanova Z, et al. Enhancer decommissioning imposes an epigenetic barrier to sensory hair cell regeneration. Dev Cell. 2021; Sep. 56(17):2471–85.108. Mellado Lagarde MM, Wan G, Zhang L, Gigliello AR, McInnis JJ, Zhang Y, et al. Spontaneous regeneration of cochlear supporting cells after neonatal ablation ensures hearing in the adult mouse. Proc Natl Acad Sci U S A. 2014; Nov. 111(47):16919–24.109. Kubota M, Scheibinger M, Jan TA, Heller S. Greater epithelial ridge cells are the principal organoid-forming progenitors of the mouse cochlea. Cell Rep. 2021; Jan. 34(3):108646.110. Udagawa T, Atkinson PJ, Milon B, Abitbol JM, Song Y, Sperber M, et al. Lineage-tracing and translatomic analysis of damage-inducible mitotic cochlear progenitors identifies candidate genes regulating regeneration. PLoS Biol. 2021; Nov. 19(11):e3001445.111. Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000; Jun. 3(6):580–6.112. Liu Z, Fang J, Dearman J, Zhang L, Zuo J. In vivo generation of immature inner hair cells in neonatal mouse cochleae by ectopic Atoh1 expression. PLoS One. 2014; 9(2):e89377.113. Iyer AA, Hosamani I, Nguyen JD, Cai T, Singh S, McGovern MM, et al. Cellular reprogramming with ATOH1, GFI1, and POU4F3 implicate epigenetic changes and cell-cell signaling as obstacles to hair cell regeneration in mature mammals. Elife. 2022; Nov. 11:e79712.114. Liu Z, Dearman JA, Cox BC, Walters BJ, Zhang L, Ayrault O, et al. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J Neurosci. 2012; May. 32(19):6600–10.115. Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005; Mar. 11(3):271–6.116. Matern MS, Milon B, Lipford EL, McMurray M, Ogawa Y, Tkaczuk A, et al. GFI1 functions to repress neuronal gene expression in the developing inner ear hair cells. Development. 2020; Sep. 147(17):dev186015.117. Jen HI, Singh S, Tao L, Maunsell HR, Segil N, Groves AK. GFI1 regulates hair cell differentiation by acting as an off-DNA transcriptional co-activator of ATOH1, and a DNA-binding repressor. Sci Rep. 2022; May. 12(1):7793.118. Wallis D, Hamblen M, Zhou Y, Venken KJ, Schumacher A, Grimes HL, et al. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development. 2003; Jan. 130(1):221–32.119. Masuda M, Pak K, Chavez E, Ryan AF. TFE2 and GATA3 enhance induction of POU4F3 and myosin VIIa positive cells in nonsensory cochlear epithelium by ATOH1. Dev Biol. 2012; Dec. 372(1):68–80.120. Xiang M, Gan L, Li D, Chen ZY, Zhou L, O’Malley BW, et al. Essential role of POU-domain factor Brn-3c in auditory and vestibular hair cell development. Proc Natl Acad Sci U S A. 1997; Aug. 94(17):9445–50.121. Yu HV, Tao L, Llamas J, Wang X, Nguyen JD, Trecek T, et al. POU4F3 pioneer activity enables ATOH1 to drive diverse mechanoreceptor differentiation through a feed-forward epigenetic mechanism. Proc Natl Acad Sci U S A. 2021; Jul. 118(29):e2105137118.122. Costa A, Sanchez-Guardado L, Juniat S, Gale JE, Daudet N, Henrique D. Generation of sensory hair cells by genetic programming with a combination of transcription factors. Development. 2015; Jun. 142(11):1948–59.123. Chen Y, Gu Y, Li Y, Li GL, Chai R, Li W, et al. Generation of mature and functional hair cells by co-expression of Gfi1, Pou4f3, and Atoh1 in the postnatal mouse cochlea. Cell Rep. 2021; Apr. 35(3):109016.124. Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, et al. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999; Jun. 284(5421):1837–41.125. Cai T, Seymour ML, Zhang H, Pereira FA, Groves AK. Conditional deletion of Atoh1 reveals distinct critical periods for survival and function of hair cells in the organ of Corti. J Neurosci. 2013; Jun. 33(24):10110–22.126. Erkman L, McEvilly RJ, Luo L, Ryan AK, Hooshmand F, O’Connell SM, et al. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996; Jun. 381(6583):603–6.127. Wiwatpanit T, Lorenzen SM, Cantu JA, Foo CZ, Hogan AK, Marquez F, et al. Trans-differentiation of outer hair cells into inner hair cells in the absence of INSM1. Nature. 2018; Nov. 563(7733):691–5.128. Chessum L, Matern MS, Kelly MC, Johnson SL, Ogawa Y, Milon B, et al. Helios is a key transcriptional regulator of outer hair cell maturation. Nature. 2018; Nov. 563(7733):696–700.129. Garcia-Anoveros J, Clancy JC, Foo CZ, Garcia-Gomez I, Zhou Y, Homma K, et al. Tbx2 is a master regulator of inner versus outer hair cell differentiation. Nature. 2022; May. 605(7909):298–303.130. Bi Z, Li L, Ren M, Gu Y, Zhu T, Li S, et al. Development and trans-differentiation into inner hair cells require Tbx2. Natl Sci Rev. 2022; Dec. 9(12):nwac156.131. Jansson L, Kim GS, Cheng AG. Making sense of Wnt signaling-linking hair cell regeneration to development. Front Cell Neurosci. 2015; 9:66.132. Chai R, Kuo B, Wang T, Liaw EJ, Xia A, Jan TA, et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci U S A. 2012; May. 109(21):8167–72.133. Hu L, Lu J, Chiang H, Wu H, Edge AS, Shi F. Diphtheria toxin-induced cell death triggers Wnt-dependent hair cell regeneration in neonatal mice. J Neurosci. 2016; Sep. 36(36):9479–89.134. Ni W, Zeng S, Li W, Chen Y, Zhang S, Tang M, et al. Wnt activation followed by Notch inhibition promotes mitotic hair cell regeneration in the postnatal mouse cochlea. Oncotarget. 2016; Oct. 7(41):66754–68.135. Kuo BR, Baldwin EM, Layman WS, Taketo MM, Zuo J. in vivo cochlear hair cell generation and survival by coactivation of β-catenin and Atoh1. J Neurosci. 2015; Jul. 35(30):10786–98.136. Atkinson PJ, Dong Y, Gu S, Liu W, Najarro EH, Udagawa T, et al. Sox2 haploinsufficiency primes regeneration and Wnt responsiveness in the mouse cochlea. J Clin Invest. 2018; Apr. 128(4):1641–56.137. Zhang L, Fang Y, Tan F, Guo F, Zhang Z, Li N, et al. AAV-Net1 facilitates the trans-differentiation of supporting cells into hair cells in the murine cochlea. Cell Mol Life Sci. 2023; Mar. 80(4):86.138. Murray D, Horgan G, Macmathuna P, Doran P. NET1-mediated RhoA activation facilitates lysophosphatidic acid-induced cell migration and invasion in gastric cancer. Br J Cancer. 2008; Oct. 99(8):1322–9.139. Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005; Feb. 6(2):167–80.140. Samarajeewa A, Lenz DR, Xie L, Chiang H, Kirchner R, Mulvaney JF, et al. Transcriptional response to Wnt activation regulates the regenerative capacity of the mammalian cochlea. Development. 2018; Nov. 145(23):dev166579.141. Quan YZ, Wei W, Ergin V, Rameshbabu AP, Huang M, Tian C, et al. Reprogramming by drug-like molecules leads to regeneration of cochlear hair cell-like cells in adult mice. Proc Natl Acad Sci U S A. 2023; Apr. 120(17):e2215253120.142. Korrapati S, Roux I, Glowatzki E, Doetzlhofer A. Notch signaling limits supporting cell plasticity in the hair cell-damaged early postnatal murine cochlea. PLoS One. 2013; 8(8):e73276.143. Li W, Wu J, Yang J, Sun S, Chai R, Chen ZY, et al. Notch inhibition induces mitotically generated hair cells in mammalian cochleae via activating the Wnt pathway. Proc Natl Acad Sci U S A. 2015; Jan. 112(1):166–71.144. Du X, Cai Q, West MB, Youm I, Huang X, Li W, et al. Regeneration of cochlear hair cells and hearing recovery through Hes1 modulation with siRNA nanoparticles in adult guinea pigs. Mol Ther. 2018; May. 26(5):1313–26.145. McGovern MM, Zhou L, Randle MR, Cox BC. Spontaneous hair cell regeneration is prevented by increased notch signaling in supporting cells. Front Cell Neurosci. 2018; 12:120.146. Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H, et al. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron. 2013; Jan. 77(1):58–69.147. Tona Y, Hamaguchi K, Ishikawa M, Miyoshi T, Yamamoto N, Yamahara K, et al. Therapeutic potential of a gamma-secretase inhibitor for hearing restoration in a guinea pig model with noise-induced hearing loss. BMC Neurosci. 2014; May. 15:66.148. Xia M, Wu M, Zhao L, Ma J, Li W, Li H. Selective ablation of inner hair cells and subsequent in-situ hair cell regeneration in the neonatal mouse cochlea. Hear Res. 2021; Aug. 407:108275.149. Milon B, Shulman ED, So KS, Cederroth CR, Lipford EL, Sperber M, et al. A cell-type-specific atlas of the inner ear transcriptional response to acoustic trauma. Cell Rep. 2021; Sep. 36(13):109758.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- microRNA-183 is Essential for Hair Cell Regeneration after Neomycin Injury in Zebrafish
- A Promotive Effect of Low-Level Laser on Hair Cell Regeneration Following Gentamicin Induced Ototoxicity in Postnatal Organotypic Culture of Rat Utricles
- Vestibular Hair Cell Regeneration in Guinea Pig after Gentamicin Damage
- Survival of Spiral Ganglion
- Atoh1 as a Coordinator of Sensory Hair Cell Development and Regeneration in the Cochlea