Lab Med Online.
2023 Jul;13(3):212-222. 10.47429/lmo.2023.13.3.212.
Immunological Characteristics of Unvaccinated Patients with COVID-19 in the Early Pandemic Period
- Affiliations
-
- 1Department of Laboratory Medicine, SMG-SNU Boramae Medical Center, Seoul, Korea
- 2Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine, Seoul National University Hospital, Seoul, Korea
- 4Seegene Medical Foundation, Seoul, Korea
- 5Department of Laboratory Medicine 5 , National Cancer Center, Goyang, Korea
- KMID: 2552746
- DOI: http://doi.org/10.47429/lmo.2023.13.3.212
Abstract
- Background
Immune responses to infectious disease differ according to disease severity. In this study, we investigated the overall laboratory profiles, including antibody titers and cytokine levels in unvaccinated patients with Coronavirus Disease 2019 (COVID-19), according to disease severity at the initial stage during the early pandemic period.
Methods
In total, 195 unvaccinated patients with COVID-19, who were hospitalized from February to August 2020 were enrolled in this study. The patients were divided into five severity categories, and their antibody titers and cytokine levels were evaluated by collecting serial blood samples up to the first three months after infection.
Results
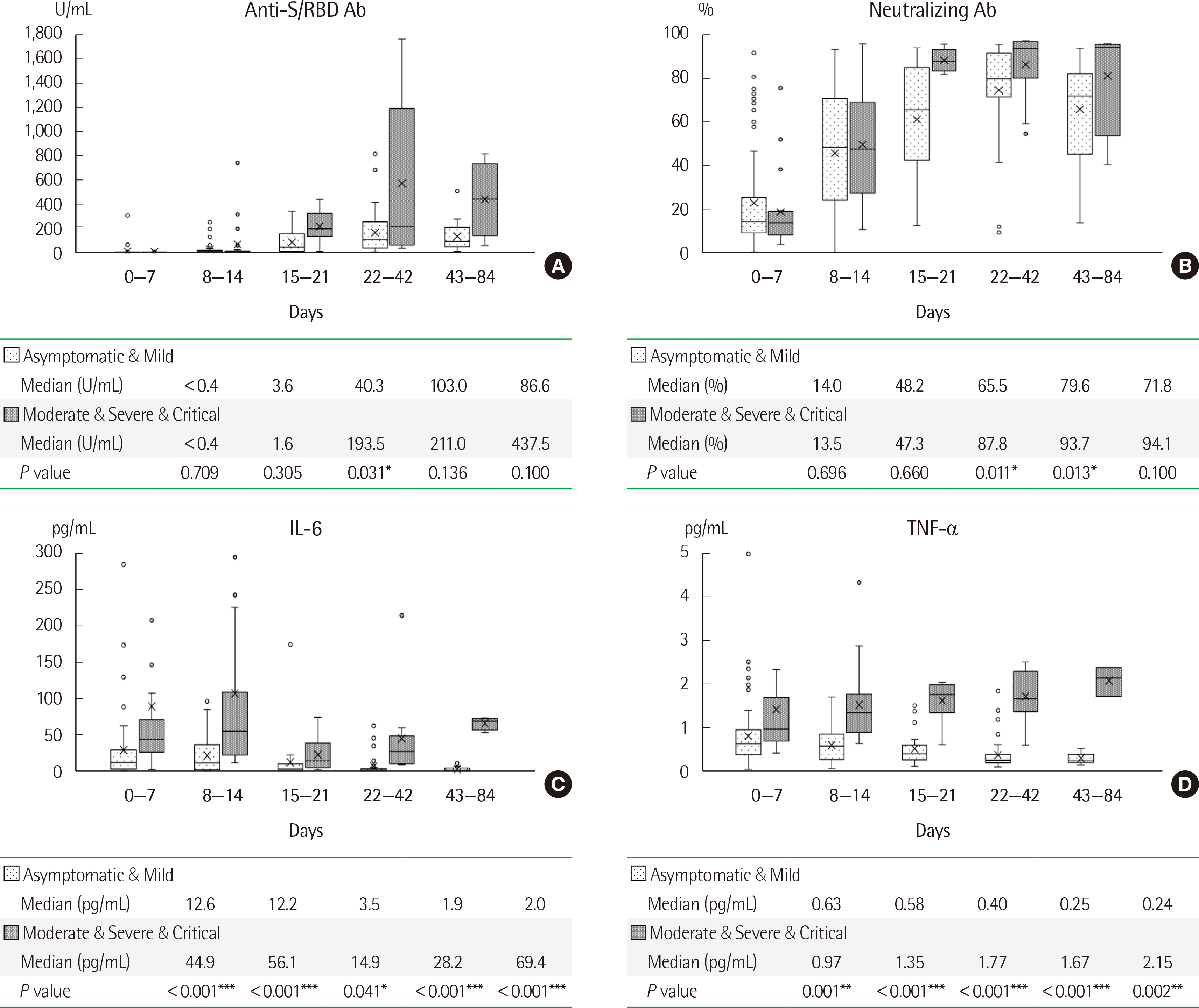
Older male patients with comorbidities had a higher probability of rapid disease progression. Patients who required intensive care showed significantly lower initial hemoglobin, platelet counts, total protein, and albumin levels, with significantly higher neutrophil-to-lymphocyte ratios, blood urea nitrogen, creatinine, and coagulation, cardiac, and inflammatory markers compared to those in patients who did not require intensive care. At three weeks after infection, the titers of both the anti-receptor binding domain of spike protein and neutralizing antibodies were significantly higher in patients with high disease severity than in patients with low disease severity; a similar trend was observed for IL-6 and TNF-α levels for at least three months from infection onset.
Conclusions
Antibody and pro-inflammatory cytokine levels showed a significant difference according to disease severity in the early stage of infection. Therefore, development of drugs targeting these antibodies and cytokines may help alleviate the severity of COVID-19.
Figure
Reference
-
1. Rothman KJ. 1976; Causes. Am J Epidemiol. 104:587–92. DOI: 10.1093/oxfordjournals.aje.a112335. PMID: 998606.2. Center for Disease Control and Prevention. Concepts of Disease Occurrence. https://www.cdc.gov/csels/dsepd/ss1978/lesson1/section8.html. Updated Nov 2011.3. Clarke KEN, Jones JM, Deng Y, Nycz E, Lee A, Iachan R, et al. 2022; Seroprevalence of infection-induced SARS-CoV-2 antibodies - United States, September 2021-February 2022. MMWR Morb Mortal Wkly Rep. 71:606–8. DOI: 10.15585/mmwr.mm7117e3. PMID: 35482574. PMCID: PMC9098232.4. World Health Organization. Coronavirus (COVID-19) Dashboard. https://covid19.who.int/. Last accessed on Oct 6th 2022.5. Kim SW, Kim SM, Kim YK, Kim JY, Lee YM, Kim BO, et al. 2021; Clinical characteristics and outcomes of COVID-19 cohort patients in Daegu metropolitan city outbreak in 2020. J Korean Med Sci. 36:e12. DOI: 10.3346/jkms.2021.36.e12. PMID: 33398946. PMCID: PMC7781854.6. Surme S, Buyukyazgan A, Bayramlar OF, Cinar AK, Copur B, Zerdali E, et al. 2021; Predictors of intensive care unit admission or mortality in patients with Coronavirus disease 2019 pneumonia in Istanbul, Turkey. Jpn J Infect Dis. 74:458–64. DOI: 10.7883/yoken.JJID.2020.1065. PMID: 33642427.7. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. 2020; Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 58:1021–8. DOI: 10.1515/cclm-2020-0369. PMID: 32286245.8. Gallo Marin B, Aghagoli G, Lavine K, Yang L, Siff EJ, Chiang SS, et al. 2021; Predictors of COVID-19 severity: a literature review. Rev Med Virol. 31:1–10. DOI: 10.1002/rmv.2146. PMID: 32845042. PMCID: PMC7855377.9. Dispinseri S, Secchi M, Pirillo MF, Tolazzi M, Borghi M, Brigatti C, et al. 2021; Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat Commun. 12:2670. DOI: 10.1038/s41467-021-22958-8. PMID: 33976165. PMCID: PMC8113594.10. Maeda T, Kashiwagi K, Yoshizawa S, Sato T, Aoki K, Ishii Y, et al. 2021; Early anti-SARS-CoV-2 immunoglobulin G response may be associated with disease severity in patients with COVID-19. Jpn J Infect Dis. 74:560–2. DOI: 10.7883/yoken.JJID.2020.799. PMID: 33642431.11. Kwon JS, Kim JY, Kim MC, Park SY, Kim BN, Bae S, et al. 2020; Factors of severity in patients with COVID-19: cytokine/chemokine concentrations, viral load, and antibody responses. Am J Trop Med Hyg. 103:2412–8. DOI: 10.4269/ajtmh.20-1110. PMID: 33124544. PMCID: PMC7695090.12. Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. 2020; SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 54:62–75. DOI: 10.1016/j.cytogfr.2020.06.001. PMID: 32513566. PMCID: PMC7265853.13. Shah VK, Firmal P, Alam A, Ganguly D, Chattopadhyay S. 2020; Overview of immune response during SARS-CoV-2 infection: lessons from the past. Front Immunol. 11:1949. DOI: 10.3389/fimmu.2020.01949. PMID: 32849654. PMCID: PMC7426442.14. Brodin P, Davis MM. 2017; Human immune system variation. Nat Rev Immunol. 17:21–9. DOI: 10.1038/nri.2016.125. PMID: 27916977. PMCID: PMC5328245.15. Marshall JS, Warrington R, Watson W, Kim HL. 2018; An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol. 14(Suppl 2):49. DOI: 10.1186/s13223-018-0278-1. PMID: 30263032. PMCID: PMC6156898.16. Cooper NR, Nemerow GR. 1984; The role of antibody and complement in the control of viral infections. J Invest Dermatol. 83:121s–7s. DOI: 10.1111/1523-1747.ep12281847. PMID: 6376646.17. Brady LJ. 2005; Antibody-mediated immunomodulation: a strategy to improve host responses against microbial antigens. Infect Immun. 73:671–8. DOI: 10.1128/IAI.73.2.671-678.2005. PMID: 15664904. PMCID: PMC547018.18. Jalkanen P, Kolehmainen P, Häkkinen HK, Huttunen M, Tähtinen PA, Lundberg R, et al. 2021; COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat Commun. 12:3991. DOI: 10.1038/s41467-021-24285-4. PMID: 34183681. PMCID: PMC8239026.19. Doria-Rose N, Suthar MS, Makowski M, O'Connell S, McDermott AB, Flach B, et al. 2021; Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 384:2259–61. DOI: 10.1056/NEJMc2103916. PMID: 33822494. PMCID: PMC8524784.20. Kim N, Minn D, Park S, Roh EY, Yoon JH, Park H, et al. 2021; Positivity of SARS-CoV-2 antibodies among Korean healthy healthcare workers 1 and 2 weeks after second dose of Pfizer-BioNTech vaccination. J Korean Med Sci. 36:e158. DOI: 10.3346/jkms.2021.36.e158. PMID: 34060264. PMCID: PMC8167405.21. Yang L, Liu W, Yu X, Wu M, Reichert JM, Ho M. 2020; COVID-19 antibody therapeutics tracker: a global online database of antibody therapeutics for the prevention and treatment of COVID-19. Antib Ther. 3:205–12. DOI: 10.1093/abt/tbaa020. PMID: 33215063. PMCID: PMC7454247.22. Deb P, Molla MMA, Saif-Ur-Rahman KM. 2021; An update to monoclonal antibody as therapeutic option against COVID-19. Biosaf Health. 3:87–91. DOI: 10.1016/j.bsheal.2021.02.001. PMID: 33585808. PMCID: PMC7872849.23. Zheng J, Deng Y, Zhao Z, Mao B, Lu M, Lin Y, et al. 2022; Characterization of SARS-CoV-2-specific humoral immunity and its potential applications and therapeutic prospects. Cell Mol Immunol. 19:150–7. DOI: 10.1038/s41423-021-00774-w. PMID: 34645940. PMCID: PMC8513558.24. Dinarello CA. 2007; Historical insights into cytokines. Eur J Immunol. 37(Suppl 1):S34–45. DOI: 10.1002/eji.200737772. PMID: 17972343. PMCID: PMC3140102.25. Zhang J-M, An J. 2007; Cytokines, inflammation, and pain. Int Anesthesiol Clin. 45:27–37. DOI: 10.1097/AIA.0b013e318034194e. PMID: 17426506. PMCID: PMC2785020.26. Beltra JC, Decaluwe H. 2016; Cytokines and persistent viral infections. Cytokine. 82:4–15. DOI: 10.1016/j.cyto.2016.02.006. PMID: 26907634.27. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. 2020; The COVID-19 cytokine storm; what we know so far. Front Immunol. 11:1146. DOI: 10.3389/fimmu.2020.01446. PMID: 32612617. PMCID: PMC7308649.28. Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, et al. 2020; An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 26:1636–43. DOI: 10.1038/s41591-020-1051-9. PMID: 32839624. PMCID: PMC7869028.29. National Institutes of Health. Clinical Spectrum of SARS-CoV-2 Infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. Last updated on Sep 2022.30. Gandhi RT, Lynch JB, Del Rio C. 2020; Mild or moderate Covid-19. N Engl J Med. 383:1757–66. DOI: 10.1056/NEJMcp2009249. PMID: 32329974.31. Korea Disease Control and Prevention Agency. 2022. Coronavirus (COVID-19) guideline, Republic of Korea (local government). 13rd-1 ed. Central Disaster Management Headquarters and Central Disease Control Headquarters.32. Center for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: Information for healthcare professionals. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. Last accessed on Feb 2022.33. Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, et al. 2020; Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2:1069–76. DOI: 10.1007/s42399-020-00363-4. PMID: 32838147. PMCID: PMC7314621.34. Basheer M, Saad E, Assy N. 2022; The cytokine storm in COVID-19: the strongest link to morbidity and mortality in the current epidemic. COVID. 2:540–52. DOI: 10.3390/covid2050040.35. Niedźwiedzka-Rystwej P, Majchrzak A, Kurkowska S, Małkowska P, Sierawska O, Hrynkiewicz R, et al. 2022; Immune signature of COVID-19: In-depth reasons and consequences of the cytokine storm. Int J Mol Sci. 23:4545. DOI: 10.3390/ijms23094545. PMID: 35562935. PMCID: PMC9105989.36. Jing X, Xu M, Song D, Yue T, Wang Y, Zhang P, et al. 2022; Association between inflammatory cytokines and anti-SARS-CoV-2 antibodies in hospitalized patients with COVID-19. Immun Ageing. 19:12. DOI: 10.1186/s12979-022-00271-2. PMID: 35248063. PMCID: PMC8897556.37. Schultheiß C, Willscher E, Paschold L, Gottschick C, Klee B, Henkes SS, et al. 2022; The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. 3:100663. DOI: 10.1016/j.xcrm.2022.100663. PMID: 35732153. PMCID: PMC9214726.38. Ling L, Chen Z, Lui G, Wong CK, Wong WT, Ng RWY, et al. 2021; Longitudinal cytokine profile in patients with mild to critical COVID-19. Front Immunol. 12:763292. DOI: 10.3389/fimmu.2021.763292. PMID: 34938289. PMCID: PMC8685399.39. Do TND, Claes S, Schols D, Neyts J, Jochmans D. 2022; SARS-CoV-2 virion infectivity and cytokine production in primary human airway epithelial cells. Viruses. 14:951. DOI: 10.3390/v14050951. PMID: 35632693. PMCID: PMC9144593.40. Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, et al. 2022; Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 399:1303–12. DOI: 10.1016/S0140-6736(22)00462-7. PMID: 35305296.41. Servellita V, Syed AM, Morris MK, Brazer N, Saldhi P, Garcia-Knight M, et al. 2022; Neutralizing immunity in vaccine breakthrough infections from the SARS-CoV-2 Omicron and Delta variants. Cell. 185:1539–48.e5. DOI: 10.1016/j.cell.2022.03.019. PMID: 35429436. PMCID: PMC8930394.42. Korea Disease Control and Prevention Agency. 2021; July 2021 status and characteristics of the COVID-19 variant virus outbreak in the Republic of Korea. Public Health Wkly Rep. 14:3388–96.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Monthly Characteristics of Adolescent Suicide Attempts Before, During and After COVID-19
- The effect of the COVID-19 pandemic on the trends and characteristics of natural and unnatural deaths in an urban Sri Lankan cohort viewed through retrospective analysis of forensic death investigations from 2019 to 2022
- The Management of Thyroid Disease in COVID-19 Pandemic
- The COVID-19 pandemic's impact on prostate cancer screening and diagnosis in Korea
- Differences in Clinical Characteristics and Antibiotic Susceptibility in Bacterial Skin Infections Before and After the COVID-19 Pandemic