Lab Med Online.
2023 Jul;13(3):205-211. 10.47429/lmo.2023.13.3.205.
Factors Associated with the Performance of Direct PCR Detection of Mycobacteria in Clinical Specimens: Retrospective Real-world Data
- Affiliations
-
- 1Department of Laboratory Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- KMID: 2552745
- DOI: http://doi.org/10.47429/lmo.2023.13.3.205
Abstract
- Background
Tuberculosis caused by Mycobacterium tuberculosis (MTB) remains a major health problem worldwide. Nontuberculous mycobacteria (NTM) infection is the primary cause of pulmonary disease. Currently, for early diagnosis of mycobacterial infection, direct PCR detection in clinical specimens is used. This study aimed to identify factors affecting the direct PCR detection of mycobacteria from clinical specimens.
Methods
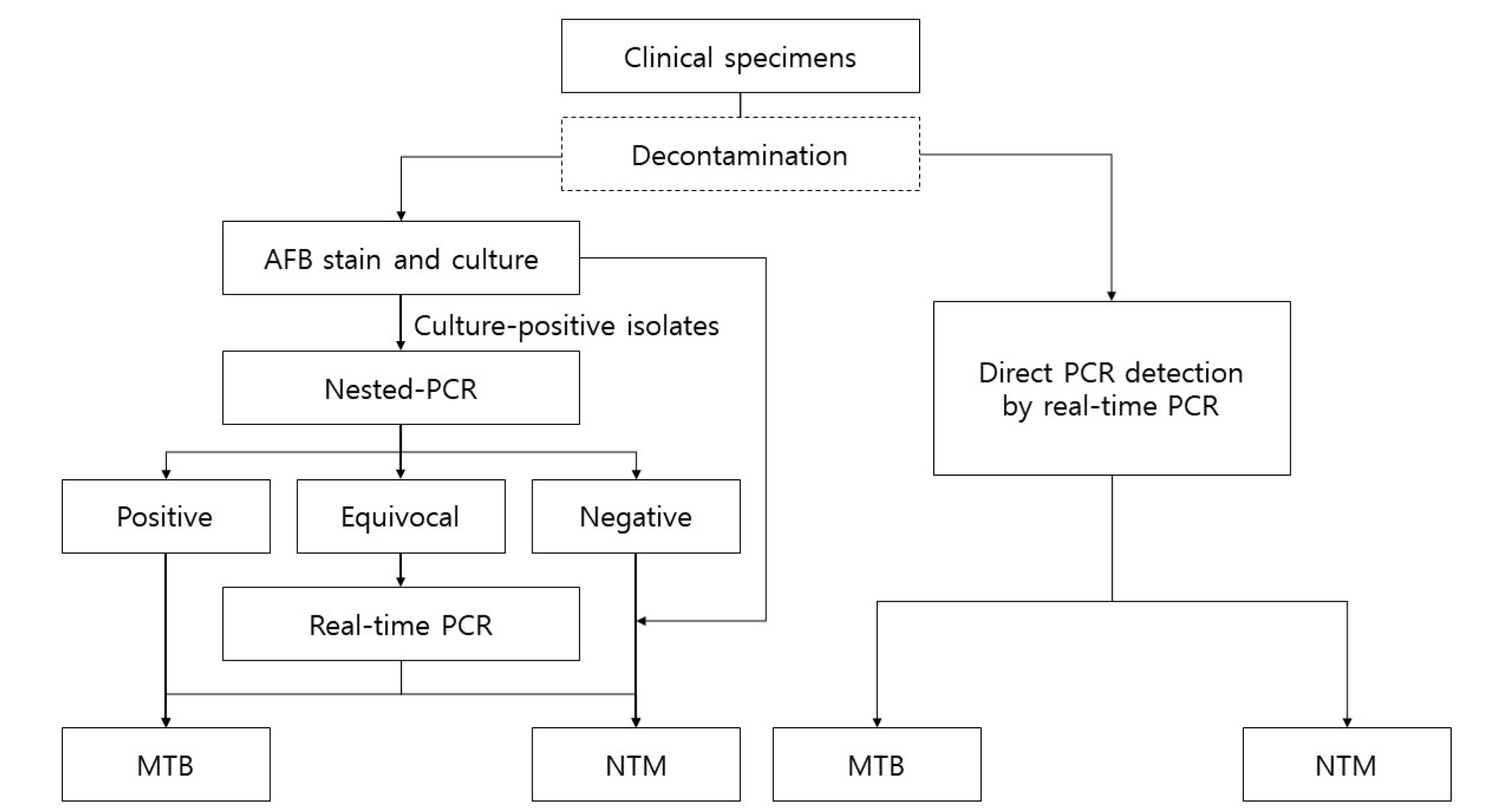
Records of mycobacterial culture from October 2016 to July 2020 were retrospectively reviewed. Using culture as the reference method, the performance of direct PCR detection was calculated. Differences in analytical performances among mycobacteria species, specimen type, and acid-fast bacillus (AFB) staining were determined using chi-squared or Fisher’s exact test.
Results
Of the 27,267 culture datasets, 1,586 datasets were selected. The sensitivity of direct PCR detection for NTM was 27.6% (95% confidence interval [ CI], 22.3–33.5) in sputum and 47.8% (95% CI, 37.3–58.5) in bronchial washing fluid (P < 0.001). The sensitivity of direct PCR detection showed higher sensitivity in smear-positive AFB than in smear-negative AFB for both MTB (93.8% vs. 51.2%; P < 0.001) and NTM (68.3% vs. 26.1%; P < 0.001).
Conclusions
AFB staining results were related with the direct PCR detection of MTB and NTM, whereas the respiratory specimen type was related with the direct PCR detection of NTM.
Keyword
Figure
Reference
-
1. World Health Organization. Global tuberculosis report 2021. https://apps.who.int/iris/rest/bitstreams/1379788/retrieve. Last accessed on August 2022.2. Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Andrejak C, et al. 2020; Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis. 71:905–13. DOI: 10.1093/cid/ciaa1125. PMID: 32797222. PMCID: PMC7768745.3. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. 2007; An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 175:367–416. DOI: 10.1164/rccm.200604-571ST. PMID: 17277290.4. Marras TK, Vinnard C, Zhang Q, Hamilton K, Adjemian J, Eagle G, et al. 2018; Relative risk of all-cause mortality in patients with nontuberculous mycobacterial lung disease in a US managed care population. Respir Med. 145:80–8. DOI: 10.1016/j.rmed.2018.10.022. PMID: 30509721. PMCID: PMC6283283.5. Park YS, Lee CH, Lee SM, Yang SC, Yoo CG, Kim YW, et al. 2010; Rapid increase of non-tuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. Int J Tuberc Lung Dis. 14:1069–71.6. Park JH, Shin S, Kim TS, Park H. 2022; Clinically refined epidemiology of nontuberculous mycobacterial pulmonary disease in South Korea: overestimation when relying only on diagnostic codes. BMC Pulm Med. 22:195. DOI: 10.1186/s12890-022-01993-1. PMID: 35562714. PMCID: PMC9107265.7. Wang HY, Kim H, Kim S, Kim DK, Cho SN, Lee H. 2015; Performance of a real-time PCR assay for the rapid identification of Mycobacterium species. J Microbiol. 53:38–46. DOI: 10.1007/s12275-015-4495-8. PMID: 25557479.8. Cho WH, Won EJ, Choi HJ, Kee SJ, Shin JH, Ryang DW, et al. 2015; Comparison of AdvanSure TB/NTM PCR and COBAS TaqMan MTB PCR for detection of Mycobacterium tuberculosis complex in routine clinical practice. Ann Lab Med. 35:356–61. DOI: 10.3343/alm.2015.35.3.356. PMID: 25932446. PMCID: PMC4390706.9. Kim J, Choi Q, Kim JW, Kim SY, Kim HJ, Park Y, et al. 2020; Comparison of the Genedia MTB/NTM Detection Kit and Anyplex plus MTB/NTM Detection Kit for detection of Mycobacterium tuberculosis complex and nontuberculous mycobacteria in clinical specimens. J Clin Lab Anal. 34:e23021. DOI: 10.1002/jcla.23021.10. Cheng VC, Yew WW, Yuen KY. 2005; Molecular diagnostics in tuberculosis. Eur J Clin Microbiol Infect Dis. 24:711–20. DOI: 10.1007/s10096-005-0039-1. PMID: 16283213.11. Drobniewski FA, Caws M, Gibson A, Young D. 2003; Modern laboratory diagnosis of tuberculosis. Lancet Infect Dis. 3:141–7. DOI: 10.1016/S1473-3099(03)00544-9. PMID: 12614730.12. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. Am J Respir Crit Care Med. 2000; 161:1376–95. DOI: 10.1164/ajrccm.161.4.16141. PMID: 10764337.13. Kim YJ, Park MY, Kim SY, Cho SA, Hwang SH, Kim HH, et al. 2008; Evaluation of the performances of AdvanSure TB/NTM real time PCR kit for detection of mycobacteria in respiratory specimens. Korean J Lab Med. 28:34–8. DOI: 10.3343/kjlm.2008.28.1.34. PMID: 18309253.14. Kim JU, Ryu DS, Cha CH, Park SH. 2018; Paradigm for diagnosing mycobacterial disease: direct detection and differentiation of Mycobacterium tuberculosis complex and non-tuberculous mycobacteria in clinical specimens using multiplex real-time PCR. J Clin Pathol. 71:774–80. DOI: 10.1136/jclinpath-2017-204945. PMID: 29559518.15. Yoo JW, Jo KW, Kim MN, Lee SD, Kim WS, Kim DS, et al. 2012; Increasing trend of isolation of non-tuberculous mycobacteria in a tertiary university hospital in South Korea. Tuberc Respir Dis (Seoul). 72:409–15. DOI: 10.4046/trd.2012.72.5.409. PMID: 23101005. PMCID: PMC3475469.16. Lee SK, Lee EJ, Kim SK, Chang J, Jeong SH, Kang YA. 2012; Changing epidemiology of nontuberculous mycobacterial lung disease in South Korea. Scand J Infect Dis. 44:733–8. DOI: 10.3109/00365548.2012.681695. PMID: 22720876.17. Weiss CH, Glassroth J. 2012; Pulmonary disease caused by nontuberculous mycobacteria. Expert Rev Respir Med. 6:597–612. DOI: 10.1586/ers.12.58. PMID: 23234447.18. Shin S, Yoo IY, Shim HJ, Kang OK, Jhun BW, Koh WJ, et al. 2020; Diagnostic performance of the GENEDIA MTB/NTM Detection Kit for detecting Mycobacterium tuberculosis and nontuberculous mycobacteria with sputum specimens. Ann Lab Med. 40:169–73. DOI: 10.3343/alm.2020.40.2.169. PMID: 31650734. PMCID: PMC6822004.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of Anyplex plus MTB/NTM and AdvanSure TB/NTM for the Detection of Mycobacterium tuberculosis and Nontuberculous Mycobacteria
- Evaluation of the AdvanSure TB/NTM Plus Real-Time PCR Assay for the Simultaneous Detection of Mycobacterium tuberculosis and Nontuberculous Mycobacteria from Clinical Specimens
- Evaluation of the Diagnostic Performance of the AdvanSure TB/NTM Real-Time PCR Kit for Detection of Mycobacteria
- Evaluation of MolecuTech Real MTB-ID for MTB/NTM Detection Using Direct Specimens
- Evaluation of Peptide Nucleic Acid Probe-based Real-time PCR for Detection of Mycobacterium tuberculosis Complex and Nontuberculous Mycobacteria in Respiratory Specimens